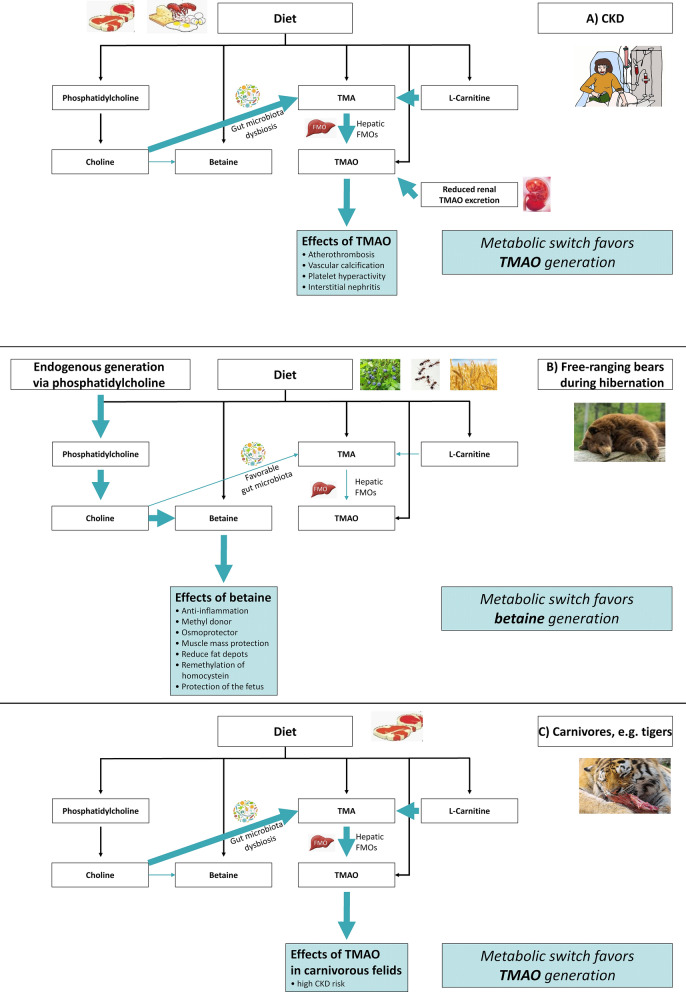
Figure 1.
Proposed regulation pattern of betaine, choline, and TMAO in (A) patients with chronic kidney disease (CKD); (B) CKD-protected, hibernating, free-ranging brown bears; and (C) CKD-prone carnivorous felids, e.g. tigers. In patients with CKD (A), high levels of trimethylamine (TMA) N-oxide (TMAO) and choline are detectable, whereas betaine levels are low. Dietary intake of phosphatidyl-choline, betaine, TMA, TMAO, and L-Carnitine contribute to circulating metabolite levels. Gut microbiota can metabolize choline and L-Carnitine to TMA. After intestinal uptake of TMA, hepatic flavin-containing monooxygenases (FMOs) convert TMA into TMAO which exerts adverse cardiometabolic effects. TMAO levels in CKD are especially elevated due to an increased microbial TMA conversion (i.e. gut microbiota dysbiosis), as well as a reduced renal excretion of TMAO. In hibernating, free-ranging brown bears (B), there is no food intake and betaine, choline, and TMAO are endogenously produced. Based on our data, we propose a metabolic switch favouring betaine rather than TMAO synthesis due to increased betaine conversion from choline and reduced gut microbiota-caused TMA production. In carnivorous animals, e.g. tigers (C), dietary intake of phosphatidylcholine, betaine, TMA, TMAO, and L-Carnitine contribute to circulating metabolite levels. Similar to patients with CKD, a metabolic switch favours TMA production by gut microbiota resulting in high TMAO levels that might be involved in the high CKD prevalence of carnivorous felids. Arrow size depicts increased or decreased activation of the respective pathway.