Figure 1. NKTs can be genetically modified to express an HLA-A2–restricted Tyrosinase-specific TCR (Tyr-TCR).
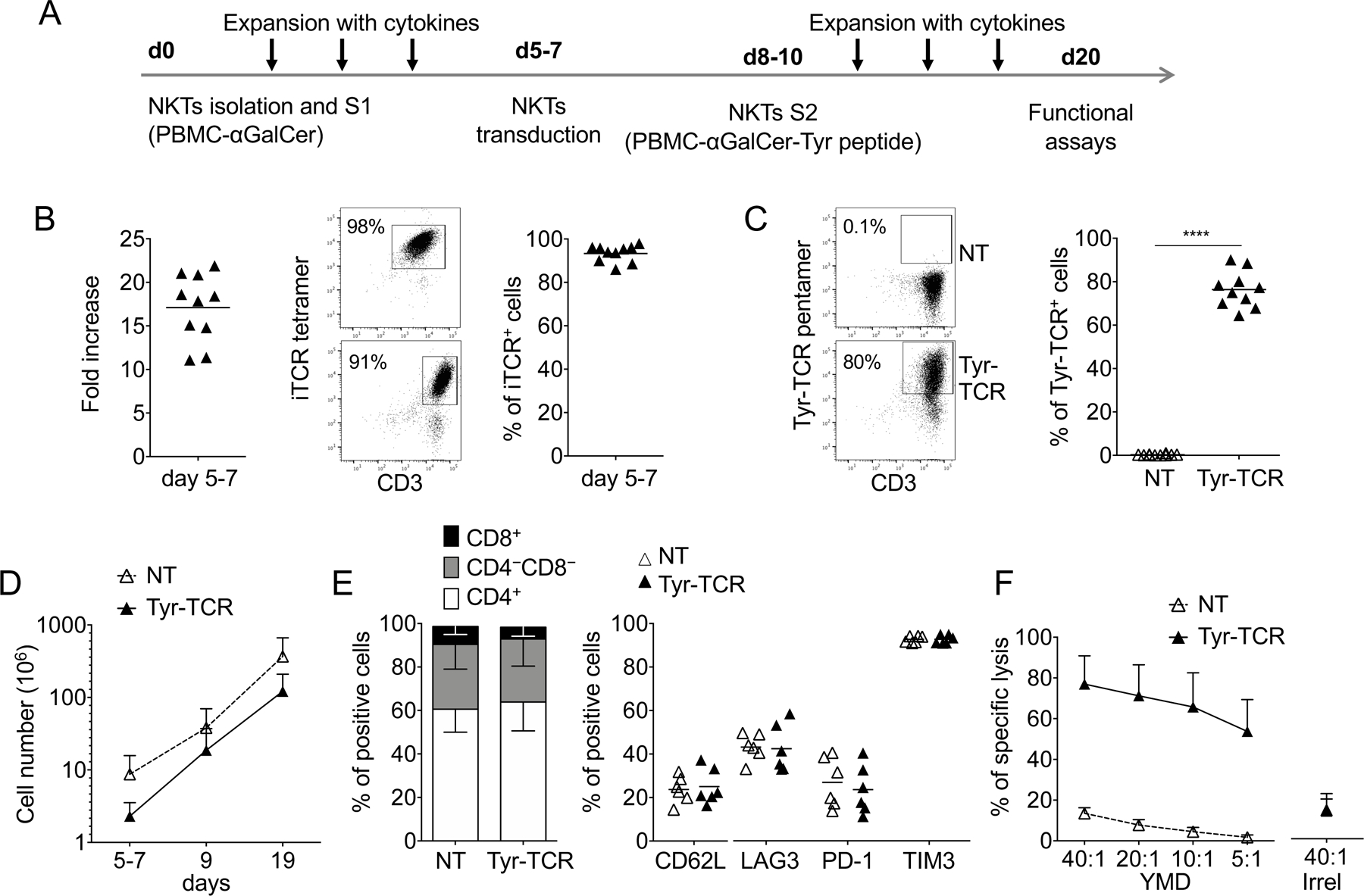
A. Schematic timeline of the protocol used to select, transduce, and expand NKTs. S1 and S2 indicate the first and second stimulation, respectively. B. Fold increase (left), representative flow cytometry plots (middle), and purity of NKTs (right) on day 5–7 after S1 (n=10, mean is shown). C. Representative flow cytometry plots (left) and summary (right) of Tyr-TCR expression by control (NKT-NT) and transduced (Tyr-TCR NKTs) NKTs assessed at day 19 of culture (n=10, mean is shown). ****p<0.0001, paired t test. D. Total cell numbers of NT-NKTs and Tyr-TCR NKTs at day 19 (n=10, mean and SD are shown). E. Phenotypic characterization of NT-NKTs and Tyr-TCR-NKTs at day 19–20 of culture (n=6, mean is shown). F. NT-NKTs and Tyr-TCR NKTs were tested in a 5-hour 51Cr-release assay against different ratios of HLA-A2+ PHA blasts loaded with either the specific tyrosinase peptide (YMD) or an irrelevant MART-1 peptide (Irrel., ELAGIGLTV), at the concentration of 100 nM (n=4, mean and SD are shown).
