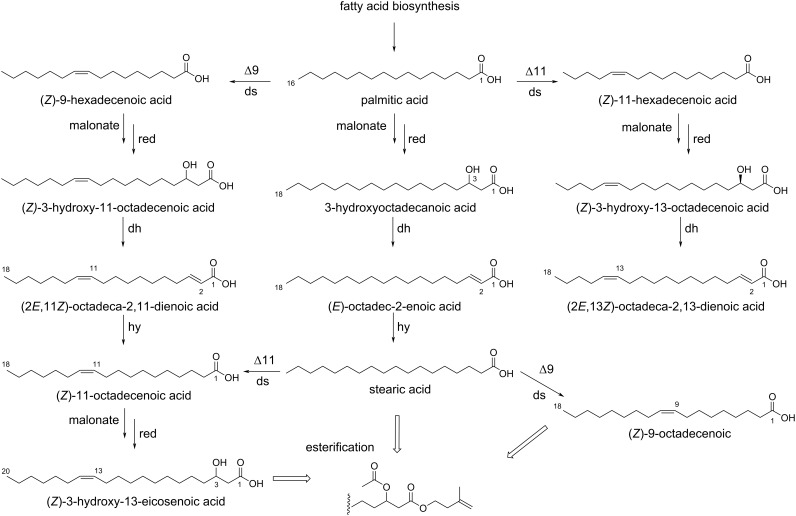
Scheme 5.
Proposed biosynthetic pathway of fatty acids leading to the observed regioisomers of the isoprenyl esters. All acids shown were found in form of their isoprenyl esters. (Z)-9-Hexadecenoic acid is obtained from palmitic acid by a Δ9-desaturase (ds). Malonate elongation and reduction (red) leads to (Z)-3-hydroxy-11-octadecenoic acid, an intermediate of the fatty acid elongation cycle. The following elimination by a dehydratase (dh) leads to (2E,11Z)-2,11-octadecadienoic acid and after hydrogenation (hy) to (Z)-11-octadecenoic acid, completing the C2-elongation. A second elongation furnishes (Z)-3-hydroxy-13-eicosenoic acid. Similarly, a Δ11-desaturase gives (Z)-11-hexadecenoic acid, (Z)-3-hydroxy-13-octadecenoic acid and (2E,13Z)-2,13-octadecadienoic acid. Both desaturases might also act on octadecanoic acid, but only the elongation of (Z)-11-octadecenoic acid can be observed, leading to (Z)-3-hydroxy-13-eicosenoic acid. The saturated 3-hydroxyoctadecanoic and stearic acids as well as (E)-2-octadecenoic acids are obtained similarly directly from palmitic acid. The proposed biosynthesis likely takes place in form of the conjugated acids, e.g., coenzyme A esters or acyl carrier proteins. Finally, the acids are converted into the isoprenyl esters and the hydroxy acids are acylated.