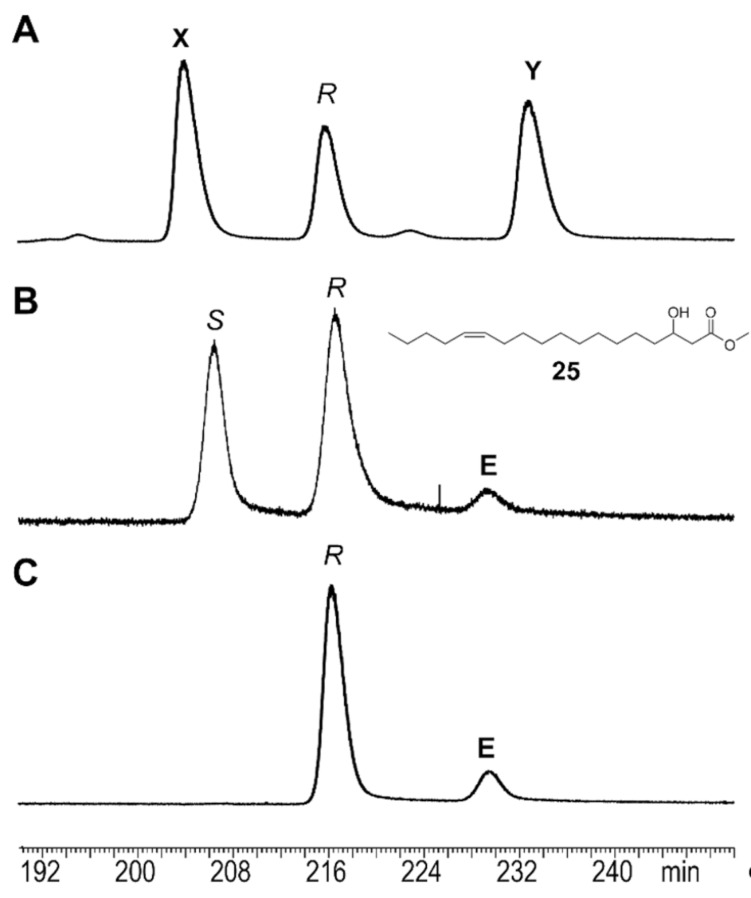
Figure 6.
Separation of the enantiomers of methyl (Z)-3-hydroxy-13-octadecenoate (25) on a β-6-TBDMS hydrodex gas chromatographic phase. A) Natural extract; B) synthetic rac-25; C) synthetic (R)-25; X: methyl 3-hydroxy-11-octadecenoate; Y: methyl 3-hydroxyoctadecanoate; E: (E)-isomer of (R)-25. The enantiomer (S,E)-25 elutes together with (R,Z)-25, indicated by the broader base of this peak in B compared to C.