Scheme 1.
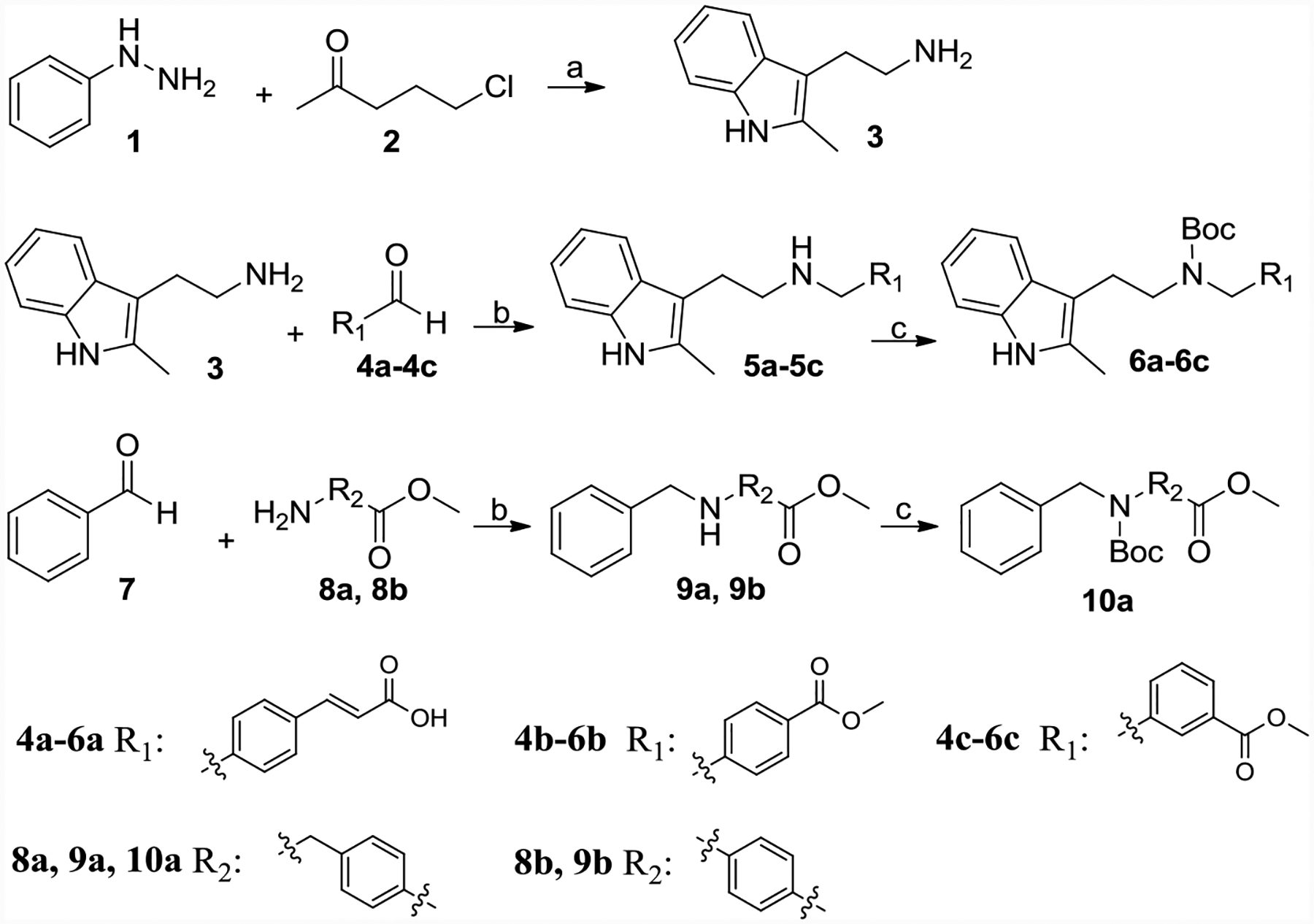
Synthesis of 3, 6a-6c, 10a and 10b.
Reagents and conditions: (a) Ethanol, NaOH, reflux, 47%; (b) NaBH3CN, CH3COOH, methanol, 70–80% yield; (c) Boc2O, TEA, DCM, 80–85% yield; (d) Propionaldehyde or benzaldehyde, NaBH3CN, HCl, Ethanol, 60–70%.
