Scheme 2.
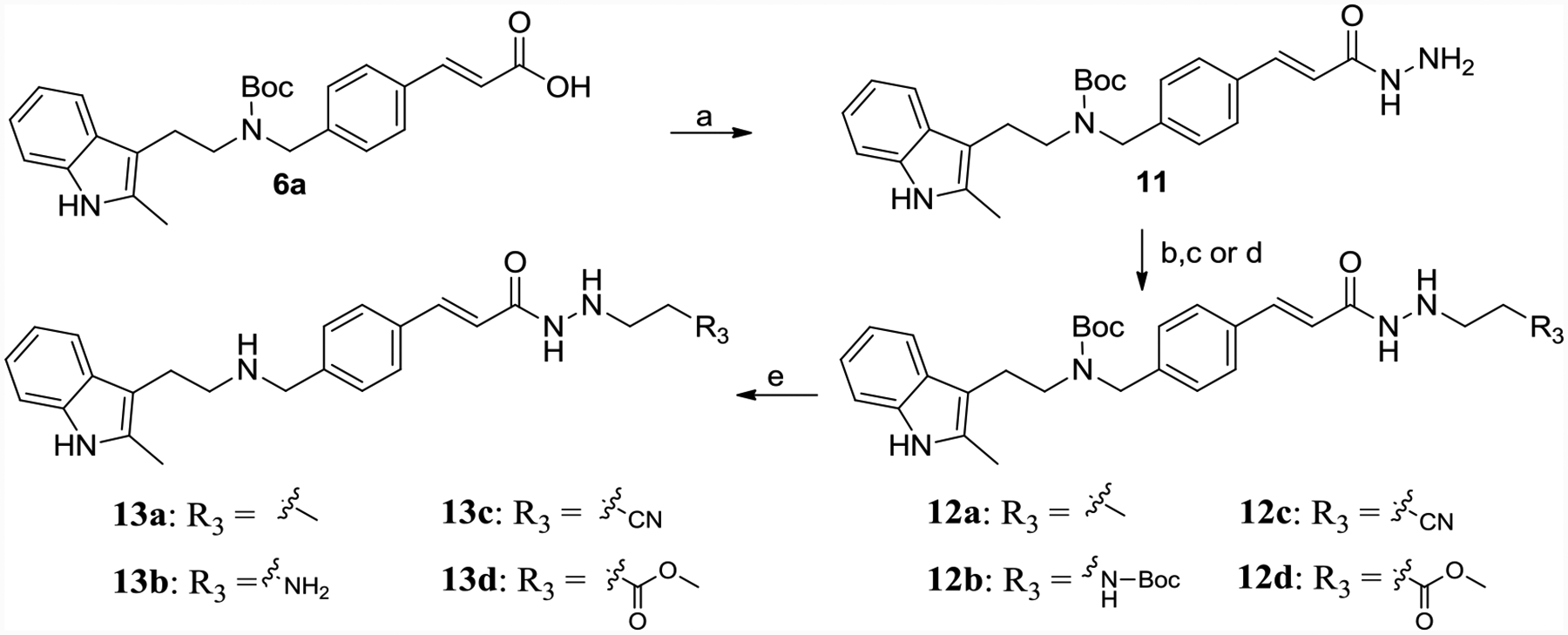
Synthesis of 13a-13d.
Reagents and conditions: (a) hydrazine monohydrate, TBTU, TEA, DMF, yield 50%; (b) propionaldehyde or N-Boc-2-aminoacetaldehyde, MgSO4, ethanol, yield 60–70%; (c) NaBH3CN, HCl, methanol, H2O, methyl orange, yield 60–70%; (d) acrylonitrile or methyl acrylate, ethanol, refluxed, yield 40–50%; (e) TFA, DCM, yield 60%.
