Figure 4.
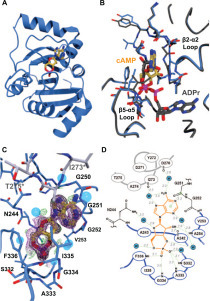
The Mac1 protein binds cAMP in an unexpected way. (A) Ribbon diagram showing the SARS-CoV-2 MaC1 domain with bound cAMP (gold sticks). (B) Overlay of the structures of the Mac1 domain with bound cAMP (gold sticks) or ADPr (gray sticks). The β2-α2 and β5-α5 loops are noted for reference. Note the difference in conformation of the β2-α2 loop (the section carrying G251). The reorientation of this loop allows it to pack against the adenine base of cAMP. (C) cAMP-binding site. The Mac1 domain protein is shown as a blue Cα trace with important residues shown as thin blue sticks. Water molecules are shown as transparent blue spheres. The stretch of amino acids shown in gray sticks represents the symmetry-related Mac1 molecule that makes contact with cAMP. The simulated annealing composite omit map is shown as a magenta mesh contoured at 1.0σ, the 2Fo-Fc map is shown in black, also at 1.0σ, and the Fo-Fc map is shown as green and red mesh at +3.0σ and –3.0σ, respectively. (D) Schematic representation of the Mac1·cAMP complex showing polar interactions between the enzyme and ligand. The cAMP ligand is shown with orange bonds. The heavy blue lines and residues drawn with black bonds represent the Mac1 protein. The heavy gray line represents the symmetry-related molecule that makes solvent-mediated contacts with cAMP. Solvent molecules are shown as blue circles with a W. Potential hydrogen bonding interactions are shown as dashed green lines with the associated distances in gray italics. The stacking interaction described in the text with the G251–G252 peptide bond is shown as a green line with light green circles at each end.
