Figure 7. Axonal Enrichment of Wnd Compartmentalizes Wnd-Dscam[TM2] Signaling.
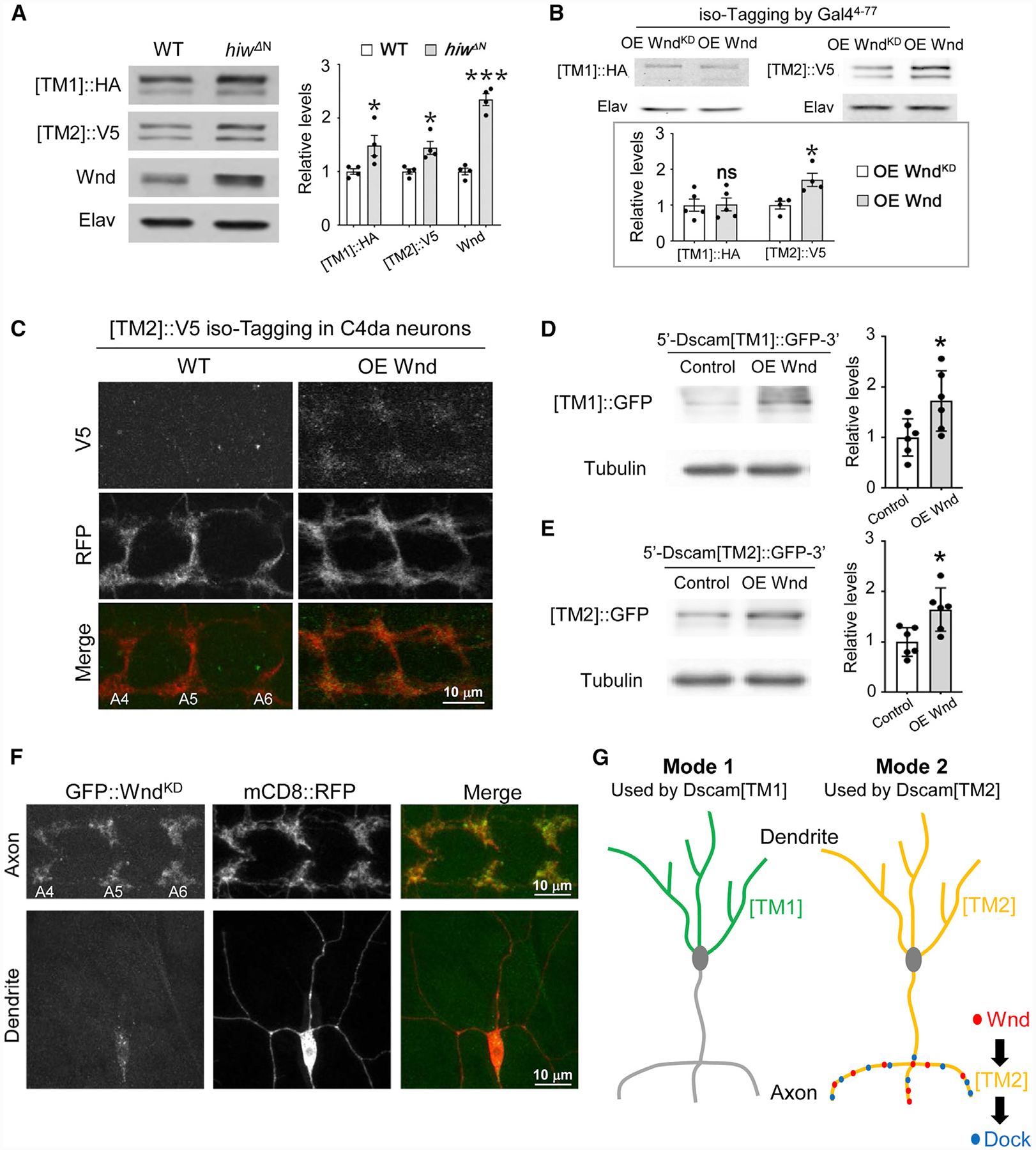
(A) Hiw suppresses the expression of both Dscam[TM1] and [TM2]. In the brains of third-instar larvae that were trans-heterozygotes of global iso-tagging of Dscam[TM1]::HA and [TM2]::V5, loss of hiw (hiwΔN) elevated the levels of both endogenous Dscam[TM1] and [TM2]. Left: western blots; right: quantification of western blots. Each dot represents the result from one independent experiment.
(B) Overexpression of Wnd increases the levels of Dscam[TM2], but not that of [TM1]. Top: the Gal44−77, which is expressed in a small set of PNS neurons and a number of CNS neurons, was used to drive the expression of R recombinase for iso-tagging and the overexpression of Wnd. Each dot represents the result of one independent experiment.
(C) Overexpression of Wnd increases endogenous Dscam[TM2] levels in the presynaptic terminals of C4 da neurons. At the third-instar larval stage, Dscam [TM2]::V5iso-tagging is no longer detectable in C4 da axon terminals. Overexpression of Wnd elevates the level of Dscam[TM2]::V5iso-tagging in these terminals.
(D and E) Wnd promotes Dscam[TM1] and [TM2] expression to a similar extent in cultured S2 cells. S2 cells were transfected with plasmids expressing Wnd and Dscam[TM1]::GFP or [TM2]::GFP with endogenous Dscam 5′ and 3′ UTR. Lysates of S2 cells were blotted by anti-GFP and anti-tubulin antibodies. Each dot represents the result of one independent experiment.
(F) Wnd is enriched in axons. GFP-tagged kinase-dead Wnd (GFP::WndKD) was expressed in C4 da neurons by ppk-Gal4. mCD8::RFP was used to label the neurons. Although the GFP::WndKD signal is enriched in axon terminals, little is observed in dendrites. Yellow arrows point to major dendritic branches.
(G) Subcellular localization expands the functional diversity of splicing isoforms via two different modes. In mode 1, the localization of an isoform in a particular subcellular location (e.g., Dscam[TM1] in dendrites) restrains this isoform from functioning in other compartments. In mode 2, the enrichment of the functional partners (e.g., Wnd and Dock) for a ubiquitously localized isoform (e.g., Dscam[TM2]) leads to isoform-specific subcellular signaling and functions.
