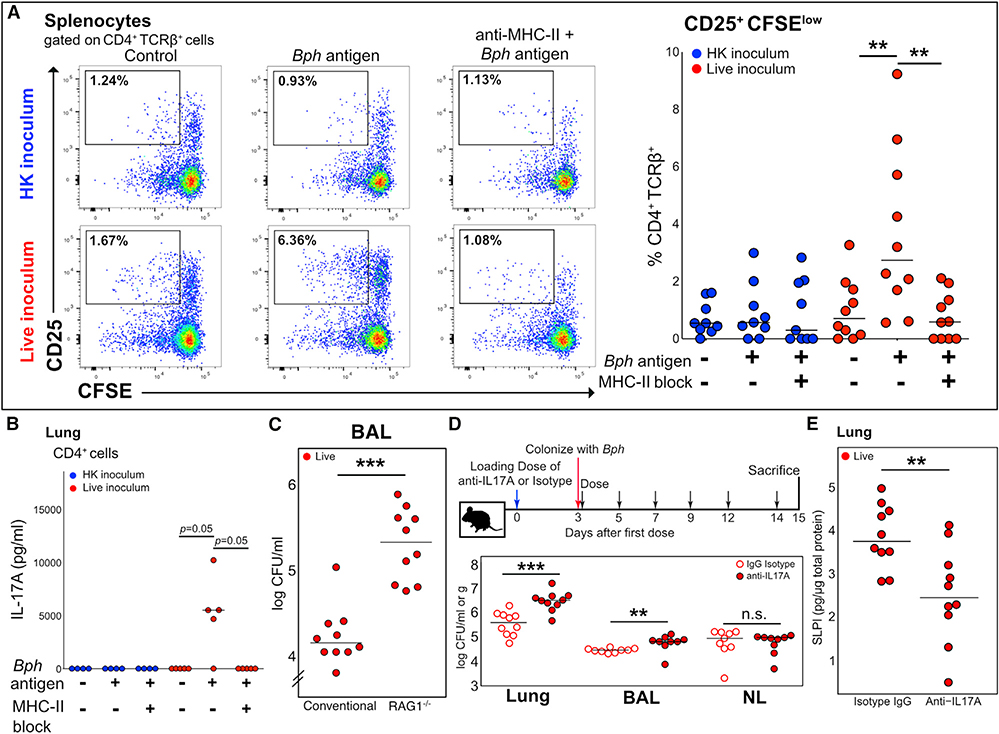
Figure 3. The Th17 Response to Bph Is Antigen Specific and Aids Controlling Colonization.
(A) Left panel: Concatenated flow cytometry plots of cultures of splenocytes of five mice taken 52 days after inoculation with either HK or live Bph. Splenocytes were loaded with either no protein or Bph proteins from a HK culture. Cells were gated on CD4+TCRβ+ cells using CD25 as an activation marker and CFSE as a proliferation marker. Representative of 2 independent experiments. Right panel: Quantification of CD25+CFSElo T cells from splenocyte cultures as shown in (A). n = 9–10 mice/group, combined from 2 independent experiments.
(B) IL-17A ELISA of culture supernatants from lung CD4+ T cells co-cultured with antigen-loaded CD11c+ dendritic cells (DCs). CD4+ T cells were isolated from the lungs of five mice taken 43 days after inoculation with either HK or live Bph.
(C) CFU of Bph recovered from BALs of WT or RAG1−/− mice 14 days after colonization. n = 10 mice/group, combined from 2 independent experiments.
(D) Top: Schematic of the experimental approach to test the role of IL-17A during Bph colonization. Bottom: CFU of Bph recovered from lung homogenates (CFU/g), BAL, or nasal lavage (CFU/ml).
(E) ELISA showing SLPI protein expression in the lungs of mice inoculated with Bph and treated with anti-IL-17A monoclonal antibody compared to the isotype control.
Statistical significance: Kruskal-Wallis followed by post hoc one-tailed paired Wilcoxon rank-sum test with adjustment for multiple hypotheses using BH correction for (A) and (B) or two-tailed Wilcoxon rank-sum test for (C)–(E). Horizontal lines indicate median values. **p < 0.01; ***p < 0.001.