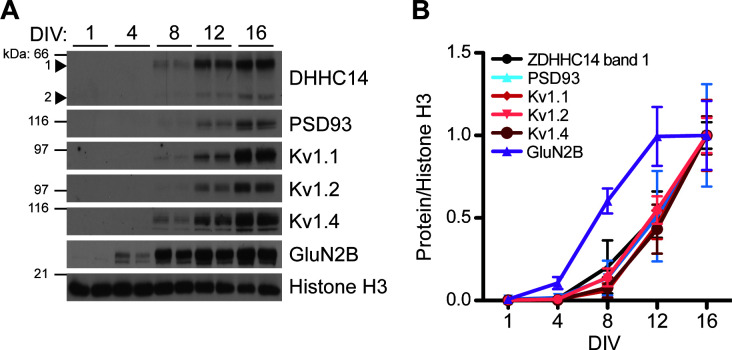
(
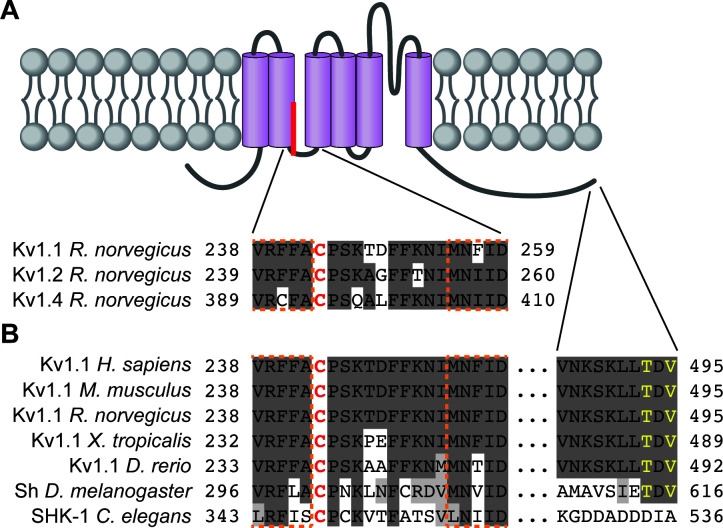
A) Schematic of a generic Kv1 channel subunit with transmembrane domains (purple) and palmitate attached to the palmitoyl-cysteine between transmembrane domains two and three. Residue numbers are indicated for the regions surrounding the palmitoyl-cysteine from rat Kv1.1 (top [
Gubitosi-Klug et al., 2005],
NP_775118.1). Corresponding regions of rat Kv1.2 and Kv1.4 are shown below (
NP_037102.1 and
NP_037103.1, respectively). (
B) Residue numbers, indicated for the regions surrounding the palmitoyl-cysteine and the 10 C-terminal amino acids from Kv1.1 orthologs, including human Kv1.1 (
H. sapiens;
NP_000208.2), mouse Kv1.1 (
M. musculus;
NP_034725.3), rat Kv1.1 (
R. norvegicus;
NP_775118.1), western clawed frog Kv1.1 (
X. tropicalis;
XP_004912858.1), Zebrafish Kv1.1 (
D. rerio;
XP_005163101.1), fruit fly Shaker (Sh;
D. melanogaster;
NP_523393.3) and roundworm SHK-1 (
C. elegans;
NP_871935.1). In
A and
B conserved residues are highlighted in dark gray, functionally conserved residues are in light gray, transmembrane domain residues are outlined in dashed orange lines, and the palmitoyl-cysteine is red. In
B PDZ-ligand consensus amino acids are in yellow.