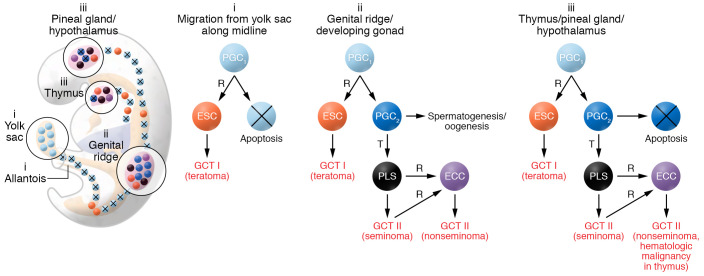
Figure 1. Model for the pathogenesis of type I and II germ cell tumors.
PGCs give origin to extragonadal GCTs. During embryogenesis (shown here at week seven), early PGCs (PGC1, light blue) migrate from the yolk sac to the genital ridge, and along the midline of the body. Early PGCs undergo apoptosis, unless, very rarely, they are reprogrammed (R) to a primed embryonic stem cell (ESC, orange), the stem cell of type I GCTs, usually benign teratomas. Type I GCTs can also develop in the genital ridge/developing gonads prior to completion of epigenetic reprogramming to late PGCs (PGC2, dark blue). Early PGCs escape apoptosis physiologically when they reach the niches in the genital ridge/developing gonad, and nonphysiologically when they lodge in the surrogate niches in the thymus or in the pineal gland/hypothalamus. In each of these niches, early PGCs can complete epigenetic reprogramming to become late PGCs/gonocytes. In the gonads their normal fate is spermatogenesis in the testis and oogenesis in the ovary. Rarely, late PGCs undergo malignant transformation (T) and form the precursor lesions for seminoma (PLS), a malignant GCT (black). A transformed late PGC/gonocyte may be reprogrammed into an ECC (purple), which is the malignant counterpart of a naive ESC, and becomes the stem cell that gives rise to the various components of a nonseminoma. In the thymus and the pineal gland/hypothalamus, late PGCs undergo apoptosis, unless they are transformed to the precursors of seminomas. Also, in these sites, transformed PGCs may be reprogrammed to ECCs, giving rise to nonseminomas, and in the thymus also hematologic malignancy.