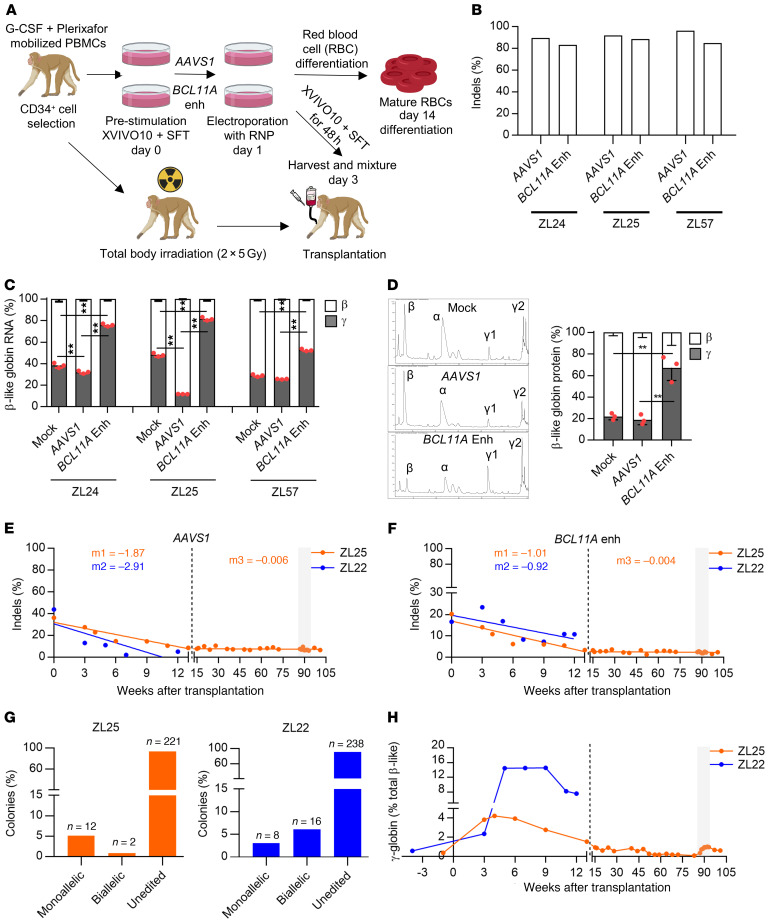
Figure 1. Durable autologous engraftment following BCL11A enhancer gene editing.
(A) Schematic representation of electroporation of rhesus CD34+ HSPCs with ribonucleoprotein (RNP) complex composed of 2×NLS SpCas9 or 3×NLS SpCas9 protein, and either BCL11A enhancer targeting (#1617) or AAVS1 targeting sgRNA. The cells are either used for ex vivo analysis or autologous transplantation. (B) Editing efficiency measured by Sanger sequencing with TIDE analysis, and (C) β-like globin RNA expression by RT-qPCR normalized to α-globin expression in nonedited (Mock), AAVS1-, and BCL11A-edited rhesus CD34+ HSPCs in small scale (ZL24 and ZL25, 5 × 104 cells, 200 pmol for both SpCas9 and sgRNAs) and large scale (ZL57, 5 × 106 cells, 1000 pmol for both SpCas9 and sgRNAs) electroporation conditions (n = 3, 1-way ANOVA followed by the Tukey’s post hoc test, **P < 0.01). (D) β-like globin protein expression by RP-HPLC (n = 3, 1-way ANOVA followed by the Tukey’s post hoc test, ** P < 0.01). ZL25 and ZL22 were transplanted with AAVS1- and BCL11A enhancer–edited cells (1:1). The gray rectangle represents the phlebotomy course. Editing frequencies in granulocytes for (E) AAVS1 and (F) BCL11A enhancer in transplanted rhesus macaques. Slopes were calculated separately for the first 13 weeks of transplantation (early progenitor phase) and later time points (HSC phase) as indicated by the dashed line. (G) Distribution of monoallelic and biallelic edited colonies collected from methylcellulose plates for bone marrow mononuclear cells of ZL25 at 100 weeks and ZL22 at 13 weeks after transplantation. (H) γ-globin protein expression percentage in ZL25 and ZL22.