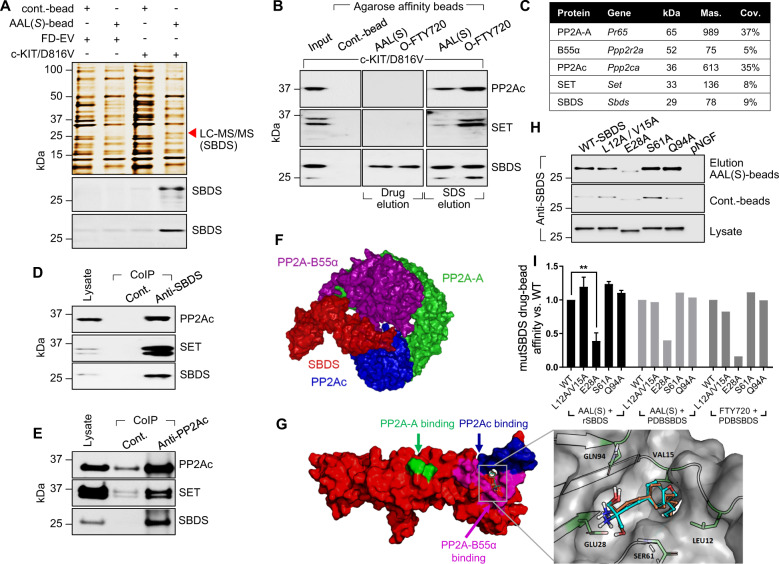
Fig. 1. SBDS is target of PP2A-activating drugs and a PP2A-interacting protein.
a Silver-stained gel of proteins pulled down by AAL(S)-drug affinity beads eluted using excess native drug (250 nM AAL(S)) in factor-dependent (GM-CSF) control myeloid progenitor cells (FD-EV) and c-KIT/D816V cells. The red arrowhead indicates the band excised and subjected to LC-MS/MS. Western blot analysis confirmed SBDS as a target of AAL(S) using both hydrophilic- (top) and hydrophobic- (bottom) AAL(S)-drug affinity beads. b Western blot confirmation of SBDS as a target of O-FTY720-beads (hydrophobic-beads) eluted using native drug (250 nM FTY720 or 250 nM AAL(S)) and further eluted using denaturing conditions (SDS). PP2Ac and SET were also eluted using reducing conditions. c Proteins identified by LC-MS/MS enriched following SBDS co-immunoprecipitation (CoIP) from c-KIT/D816V cells. d Western blot confirmation of SBDS CoIP LC-MS/MS results. e Reciprocal CoIP using PP2Ac as bait confirming SBDS–PP2A interactions. f Protein–protein docking of SBDS (red) with PP2A subunits Aα (green), B55α (purple) and Cα (blue) predicts SBDS NTD to directly bind to PP2Ac and PP2A–B55α. g Docking with SBDS indicates that AAL(S) (orange) and FTY720 (green) form hydrogen bonds (S61, Q94), a salt bridge (E28) and hydrophobic interactions (L12, V15) with the NTD of SBDS, in the region surrounded by predicted binding sites with PP2Ac (blue) and B55α (purple). h Site directed mutagenesis induced alanine substitutions; SBDS mutants, L12A/V15A, E28A, S61A and Q94A were overexpressed, purified and used in AAL(S)-drug bead affinity chromatography. SDS-PAGE gel shifting occurred in the E28A mutant to increase gel migration by the binding of additional SDS molecules, without altering the secondary structure. i Quantitative assessment of AAL(S) and FTY720 affinity for wild-type (WT) and alanine mutant SBDS using recombinant protein, and in silico alanine mutant PDB SBDS structures.