FIGURE 7. SPM adjuvant-enhanced P6 vaccination efficacy diminishes SHS-exacerbated, infection-induced BAL inflammatory cytokine profile and lung-epithelial damage.
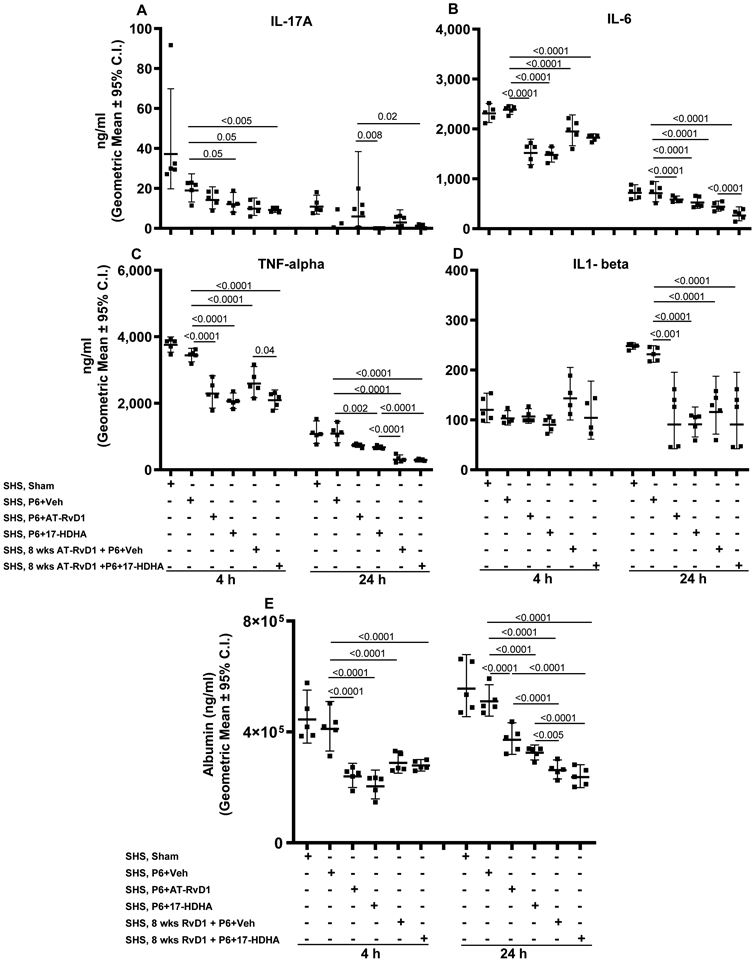
Mice exposed to chronic SHS were either untreated or treated with AT-RvD1 for 8 wks prior to vaccination with P6 ± SPM adjuvant 17-HDHA or AT-RvD1, and then infected in the lungs with acute NTHI 8 wks after the start of vaccination. Concentration of proinflammatory cytokines (A) IL-17A, (B) IL-6, (C) TNF-α and (D) IL1-β in the BAL collected at 4 and 24 h following infection were evaluated by ELISA. (E) The levels of albumin in the endpoint BAL samples as a surrogate marker of lung-epithelial damage were quantified by ELISA. All treatment groups were assayed at the same time, and data represent results generated from a single experiment using a total of 60 mice (n=5 mice for each treatment, at each time point). Data from individual mice are shown and the results are depicted as geometric mean ± 95% C.I. Overall p<0.0001 for IL-17A, IL-6, TNF-alpha, albumin (at 4 and 24 h) and for IL1-beta (at 24 h) comparing SHS, sham or SHS, P6+vehicle group vs all treatment groups with Tukey’s posttest for multiple comparisons.
