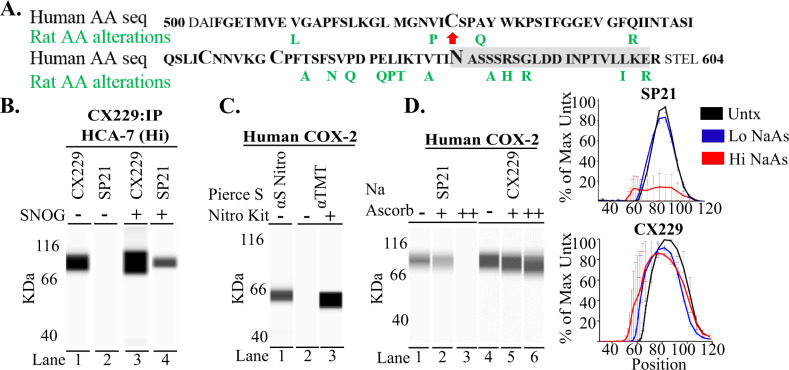
Fig. 2. SP21 recognizes S-nitrosylated COX2.
A Human and rat amino acid (AA) sequence of PTGS2 gene region used as immunogens (SP21 = black bold and CX229 = gray box). Potential posttranslational modification (larger font size) S-nitrosylation site is seen at cysteine 526 (red arrow), disulfide bond sites at AA 555 and 561, and a glycosylation site at AA 580. B COX2 immunoprecipitation (IP) of HCA-7 cell lysate using CX229 is recognized by CX229 (lane 1), but not SP21 (lane 2). On biochemical S-nitrosylation of the CX229 IP, SP21 regains reactivity to COX2 (lane 4). C Western blot analysis confirms recombinant human COX2 protein contains the S-nitrosylated form of COX2 as detected by a pan-nitrosylation specific monoclonal antibody (lane 1), as well as by assessment of nitrosylation modification using the Pierce S-nitrosylation kit and the anti-TMT antibody (lanes 2 and 3). D Western blot analysis confirms dose-escalating Na ascorbate treatment (−, +, ++) used for de-nitrosylation of recombinant human COX2 protein results in dose-dependent loss of SP21 signal (lanes 1–3). No or minimal loss of CX229 reactivity was observed after Na ascorbate treatment (lanes 4–6). Quantitation of COX2 western blots from three separate Na ascorbate experiments (right panel electrophoretograms).