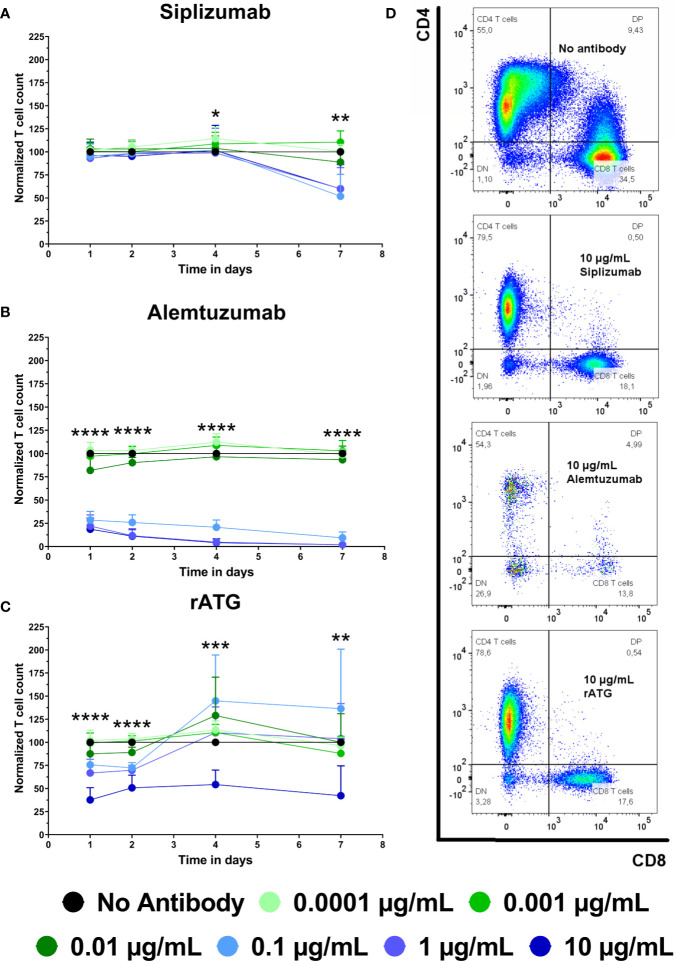
Figure 3.
The effect of siplizumab (A), Alemtuzumab (B), and rabbit anti-thymocyte globulin (rATG) (C) on T cell count in allogeneic mixed lymphocyte reaction (MLR). T cell count was measured on days 1, 2, 4, and 7. Data was normalized to T cell count in no antibody control and is displayed as the mean of all data points (N = 9 donor pairs) ± SD. T cell count was measured as number of events in the CD3+ CD56− lymphocyte gate. Data were analyzed using two-way ANOVA followed by Dunnett’s multiple comparison test with untreated controls (no antibody) serving as the comparison data set (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). (A) Siplizumab did not change normalized T cell count on days 1 to 4 of allogeneic MLR but significantly reduced T cell count after 7 days at 0.1–10 µg/ml (p ≤ 0.0082). (B) Alemtuzumab significantly reduced T cell count on all time points at 0.1, 1, and 10 µg/ml (p < 0.0001). At 0.01 µg/ml Alemtuzumab induced significant T cell depletion after 1 (p = 0.0157) and 2 (0.0036) days of allogeneic MLR. (C) After 1 and 2 days of allogeneic MLR, rATG induced significant T cell depletion at 0.01 µg/ml (p = 0.0366; p = 0.0018), 0.1 µg/ml (p = 0.0011; p < 0.0001), 1 µg/ml (p = 0.0007; p < 0.0001) and 10 µg/ml (p < 0.0001). Only 10 µg/ml rATG led to sustained depletion after 4 (p = 0.0001) and 7 (p = 0.003) days. (D) Representative dot plots illustrating differences in T cell count in MLRs treated with no antibody, 10 µg/ml siplizumab, 10 µg/ml Alemtuzumab, and 10 µg/ml rATG.