Figure 5.
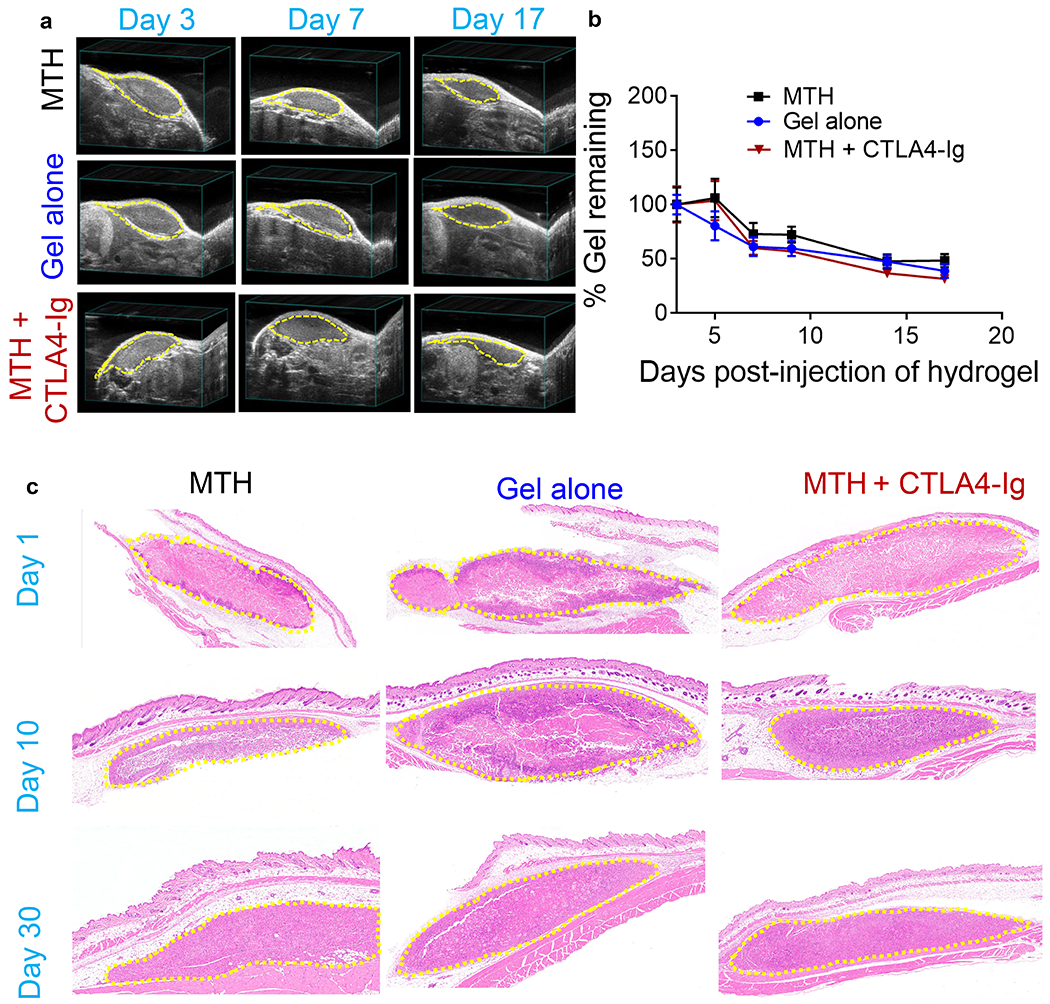
(a) Bioresorption of subcutaneously injected MTH (16 mM tofacitinib, 5% (v/v) DMSO and 1% (w/v) peptide 1), 1% (w/v) peptide 1 gel alone, and MTH co-administered with CTLA4-Ig delivered IP monitored by ultrasound at d3, 7, 17. (b) Quantitation of echograms at d3, 5, 7, 9, 14, and 17 (n = 5). (c) H&E staining of tissue sections taken at d1, 10, and 30 post injection from C57BL/6J mice that received a single subcutaneous injection of MTH, gel alone, and MTH co-administered with CTLA4-Ig delivered IP. The injection sites are outlined by yellow dash.
