Fig 3.
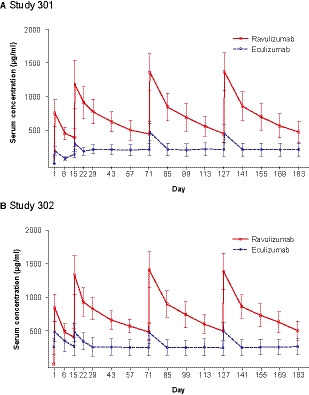
Mean (SD) serum drug concentrations over time (PK analysis population). (A) Study 301. (B) Study 302. Blood was collected for serum drug assessment (ravulizumab and eculizumab) and free and total serum C5 quantitation on day 1 at the end of infusion, and anytime on days 8, 22, 29, 43, 57, 85, 99, 113, 141, 155, and 169, for the ravulizumab group and pre‐dose for the eculizumab group; at days 15, 71, and 127 data are from pre‐dose and end of infusion for both treatment groups; and at day 183 data are from end of the primary evaluation period (prior to dosing) for both treatment groups. Lower limit of quantitation was 1·00 µg/ml for ravulizumab and 5·00 µg/ml for eculizumab. PK, pharmacokinetic; SD, standard deviation. [Colour figure can be viewed at wileyonlinelibrary.com]
