Fig. 1.
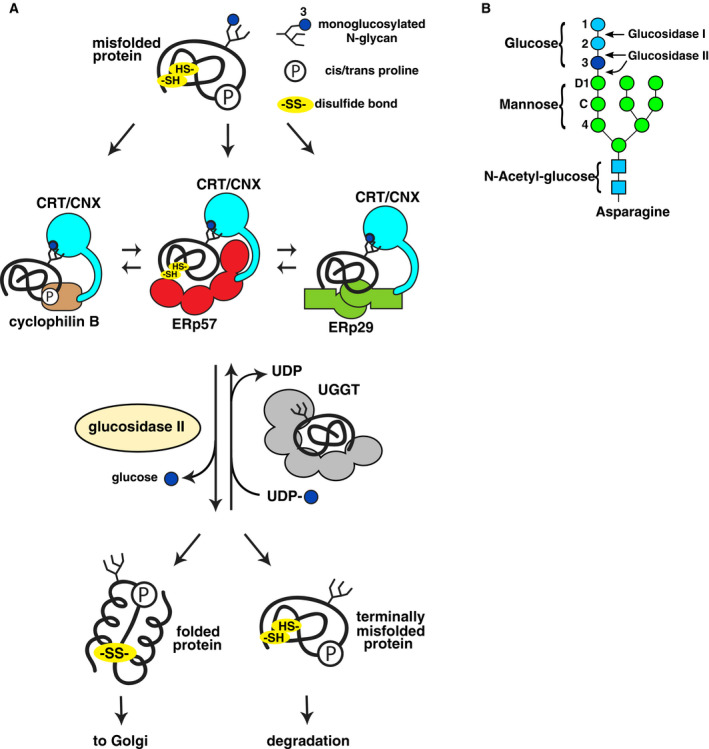
Overview of calnexin/calreticulin cycle. (A) The monoglucosylated form of newly synthesized glycoproteins proteins binds to calreticulin (CRT)/calnexin (CNX) and promotes protein folding with assistance from ERp57, CypB, and ERp29. Following release of the terminal glucose by glucosidase II, natively folded proteins are transported to Golgi. Incompletely folded proteins are reglucosylated by UGGT and rebind calreticulin/calnexin for additional folding cycles. If multiple folding cycles are unsuccessful, terminally misfolded proteins are transported to the cytoplasm for degradation via the ER‐associated protein degradation (ERAD) pathway. (B) Structure of N‐linked glycan. The precursor glycan is attached to the protein with three glucose residues. The first two are removed through the action of glucosidases I and II to generate the monoglucosylated form that is required for binding calnexin and calreticulin. UGGT acts on misfolded glycoproteins to add back glucose to the glycan for additional rounds of chaperone‐mediated folding.
