Fig. 2.
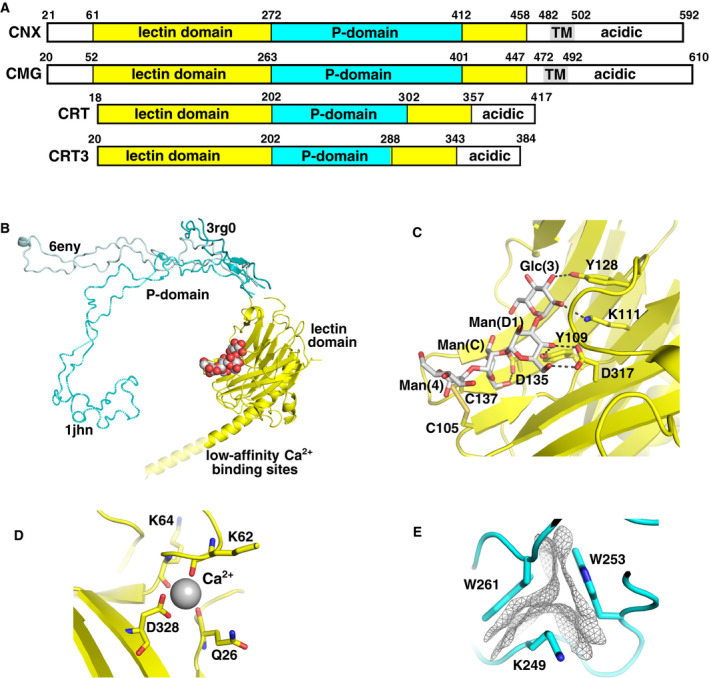
Calnexin/calreticulin structure. (A) Domain architecture of calnexin (CNX), calmegin (CMG), calreticulin (CRT), and calreticulin 3 (CRT3). The P‐domain is inserted into the lectin domain, while calnexin and calmegin also possess a transmembrane (TM) domain. The P‐domain in calnexin and calmegin is composed of four repeated modules, while the domain in calreticulin and calreticulin 3 contains only three modules. (B) Overlay of calnexin (PDB 1JHN) and calreticulin (PDB 3RG0 and 6ENY) structures illustrates flexibility of the P‐domain and the site of the glycan (red/white) bound to the lectin domain. In the peptide‐loading complex (PDB 6ENY), the C terminus of calreticulin forms a long helix, but it is unlikely to be folded in solution. The C termini of both proteins are rich in acidic residues that bind Ca2+ ions. (C) Four sugars of the glycoprotein glycan bind to the lectin domain along the β‐sheet surface (PDB 3O0W). The sugar‐binding specificity arises from the numerous hydrogen bonds between the glycan and protein. A disulfide bridge between Cys105 and Cys137 interacts with the Man(4) moiety. (D) High‐affinity Ca2+‐binding site in the lectin domain of calreticulin (PDB 3O0W). (E) Each repeated module in the P‐domain contains a small hydrophobic core of two tryptophans and a lysine residue. Residues from the calreticulin P‐domain structure (PDB 5V90) show the close packing of the hydrophobic van der Waals surfaces.
