Fig. 3.
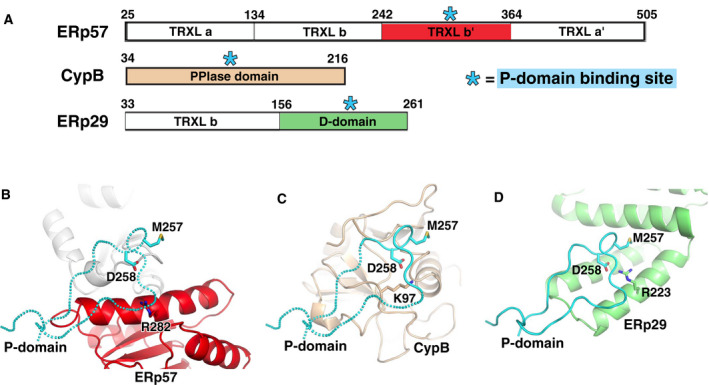
Interactions of calnexin/calreticulin with other ER proteins. (A) Domain architecture of calnexin/calreticulin‐binding partners. The asterisks mark domains required for interactions with calnexin/calreticulin. The tip of P‐domain (cyan) interacts with very different structural scaffolds from protein disulfide isomerase ERp57 (B), cis‐trans prolyl isomerase CypB (C), and general chaperone ERp29 (D). Met257 and Asp258 are part of the conserved Met‐Asp‐Gly sequence at the tip of P‐domains instrumental for the binding. Positively charged residues Arg282 (ERp57), Lys97 (CypB), and Arg223 (ERp29) indispensable for interactions with calnexin/calreticulin are also shown as sticks. Crystal structure of calreticulin P‐domain (PDB 5V90) was overlaid with cryo‐EM structure of P‐domain bound to ERp57 (PDB 6ENY) and with a shorter segment of P‐domain bound to CypB (PDB 3ICI). The modeled parts of P‐domains are shown with dashed lines. The ERp57‐P‐domain model was assembled using the high‐resolution structure of ERp57 bb′ domain fragment (PDB 2H8L) and relative orientation of both proteins in cryo‐EM structure.
