Scheme 1.
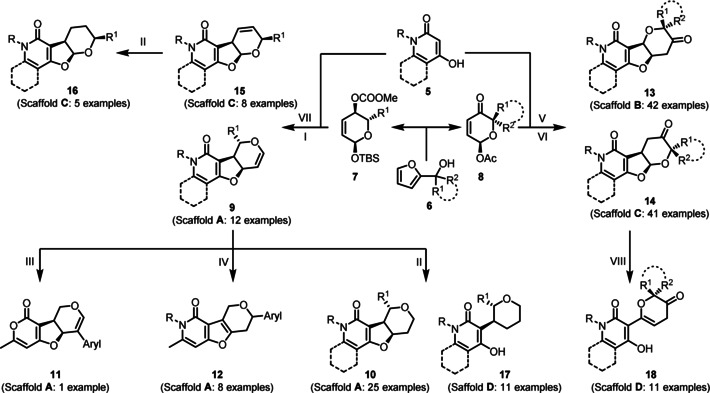
Synthesis of the PFP pseudo NP library; General reaction conditions: I) R=Me: Pd(PPh3)4, THF/DMF, rt, overnight, R=H: Pd[P[3,5‐(CF3)2C6H3]3]3, THF/DMF, 100–110 °C MW, 1–2 h; II) R=H, R=Me: Pd/C, H2, toluene, rt, 1 h–1 d; III) 1. NBS, AgNO3, acetonitrile, 80 °C, 2 h; 2. Aryl‐B(OH)2, NaOtBu, Pd(OAc)2, Xphos, toluene, 130 °C MW, 40 min; IV) Aryl‐B(OH)2, Pd(OAc)2, DMF, rt, overnight; V) R1=R2=H, R1=R2=Me, R1=R2=N‐Boc piperidine, R1=H R2=Me: Pd(PPh3)4, NEt3, THF/DMF, rt, overnight; VI) R1=R2=H, R1=R2=Me, R1=R2=N‐Boc piperidine: quinine, DCM, 60 °C, 18 h; VII) R=Me: Pd[P[3,5‐(CF3)2C6H3]3]3, THF/DMF, 100–110 °C MW, 1–2 h; VIII) R1=R2=N‐Boc piperidine: HCl in dioxane, 0 °C, rt, 90 min.
