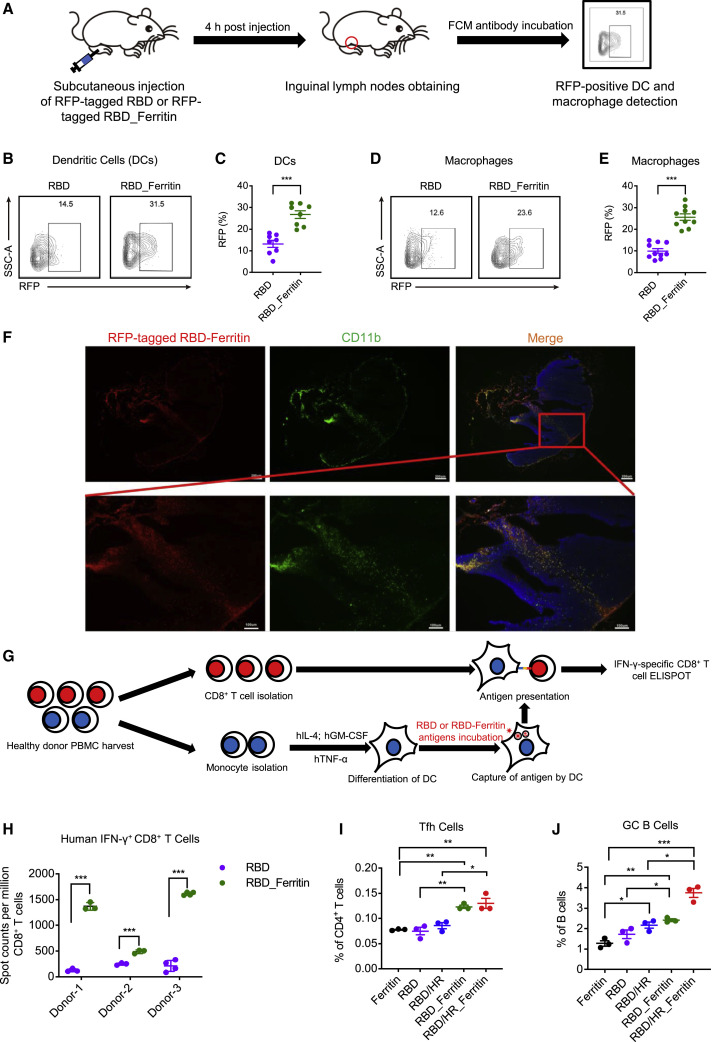
Figure 4.
Potent Antigen Presentation and T-B Coordination Induced by Nanoparticle Vaccines
(A) Schematic of in vitro DC and macrophage antigen internalization experiment. Six C57BL/6 mice were subcutaneously injected with equal moles of RFP-tagged RBD and RFP-tagged RBD-Ferritin, both proteins were adjuvanted with SAS adjuvant. 4 h post injection, inguinal lymph nodes from both sides were obtained and proceeded to FCM analysis to determine the percentages of RFP-positive DCs (B220-CD11chiMHC-II+) and macrophages (B220-CD11b+F4/80-CD169+). (B-E) The FCM results of RFP-positive DCs and macrophages.
(B) and (D) represented the typical FCM figures.
(C) and (E) represented the statistical graphs of the FCM results (n = 8 for DCs, n = 10 for macrophages).
(F) Cryosections of inguinal lymph nodes were immunostained with antibodies against CD11b. RFP-positive cells indicated RFP-tagged RBD-Ferritin nanoparticles. The blue staining indicated DAPI-stained nuclei. Scale bars in the upper panel represented 200 μm. Scale bars in the lower panel represented 100 μm.
(G) Schematic of in vitro DC antigen presentation experiments. PBMCs were harvested from three healthy individuals and proceeded to monocyte isolation. Mature DCs were induced and then loaded with RBD or RBD-Ferritin antigens, followed by co-culture with autologous CD8+ T cells. ELISpot assays were conducted for IFN-γ CD8+ T cells.
(H) ELISpot results of in vitro DC antigen presentation experiment in three healthy donors (n = 3 for Donor1 and Donor 2, n = 4 for Donor 3).
(I and J) BALB/c mice were immunized with different vaccines. Ten days post immunization, mice were euthanized. The percentages of Tfh cells (CD4+CXCR5+PD-1+) and GC B cells (CD19+B220+CD95+ GL7+) were determined by FCM (n = 3). Experiments were conducted independently in triplicates. Data represented as mean ± SEM. P values in (C), (E), and (H) were calculated by Student’s t test. Adjusted p values in (I) and (J) were calculated by one-way ANOVA with Tukey’s multiple comparisons test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
See also Figure S3.