Fig. 1. Effect of oxygen fugacity on the relative abundances of major gas species.
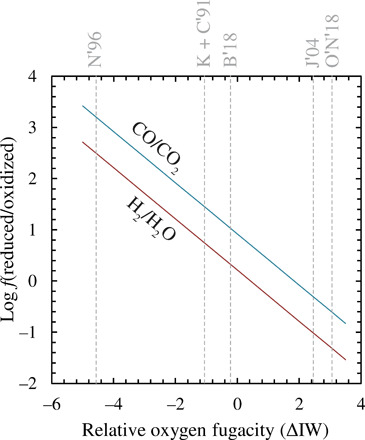
Calculated H2/H2O (red) and CO/CO2 (blue) ratios of an ideal gas at 2173 K as a function of oxygen fugacity expressed relative to the ΔIW. These ratios are independent of molar H/C of the gas phase, as well as its total pressure (provided it remains ideal), and are controlled only by temperature (which determines the intercept, corresponding to the logarithm of the equilibrium constant of reaction) and (fO2)0.5 (the exponent being the slope of the line). Dashed gray lines denote the fO2 of a peridotite liquid with a BSE composition (24) and an Fe3+/ΣFe ratio 0.037 (fig. S8) (40, 41) calculated according to different model parameterizations of the relationship between fO2 and Fe3+/Fe2+ in silicate melts; K + C′91 = Kress and Carmichael (29); N′96 = Nikolaev et al. (36); B′18 = Borisov (37); J′04 = Jayasuriya et al. (38); O′N′18 = O’Neill et al. (39).
