Fig. 2. Relationship between iron oxidation state and oxygen fugacity in quenched peridotite liquid.
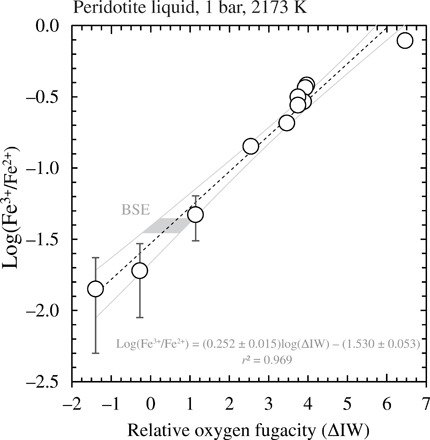
The log(XFe3+/XFe2+) ratios determined by Fe K-edge XANES in a peridotite liquid (1 bar, 2173 K) quenched to glass as a function of the imposed oxygen fugacity, expressed as the log unit deviation from the IW buffer (ΔIW). Error bars on points are the SD. The dashed black line is a linear regression through the data, and the gray curves represent the 95% confidence envelope on the regression. The shaded gray area pertains to the range of log(XFe3+/XFe2+) defined by the BSE, as inferred from Fe3+/Fe2+ ratios of whole-rock peridotites (table S6) (40, 41).
