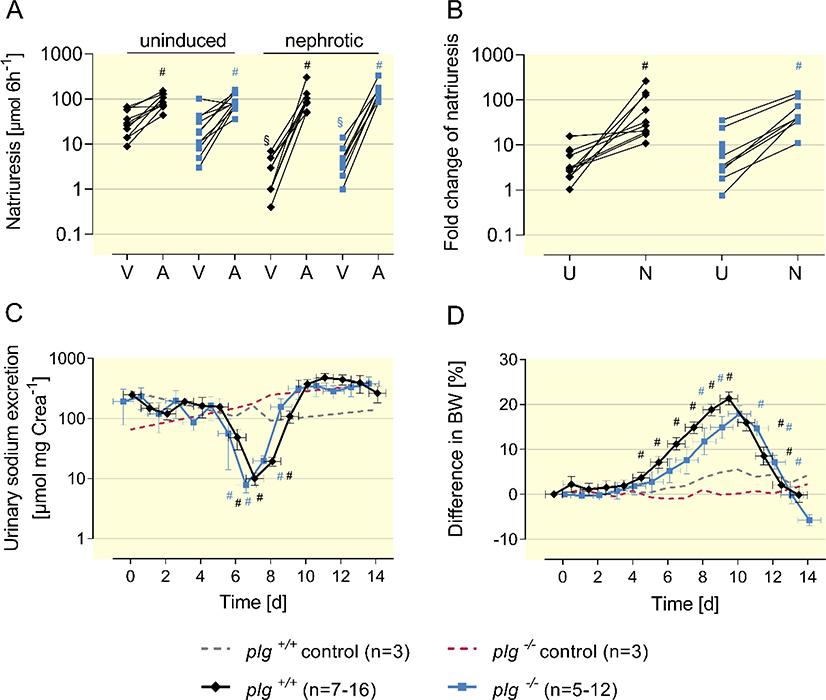
Figure 3. ENaC activation and sodium retention in nephrotic nphs2Δipod*plg+/+ and nphs2Δipod*plg−/− mice.
(A)Natriuretic response to vehicle (injectable water, 5 μl g−1 bw) or amiloride (10 μg g−1 bw i.p.). in uninduced and nephrotic nphs2Δipod*plg+/+ and nphs2Δipod*plg−/− mice. Urine was collected for 6 h after injection and all mice underwent vehicle and amiloride injection sequentially (at day −21 and day 8, respectively). Mean values were 34 ± 7 and 26 ± 9 μmol 6 h−1 in uninduced nphs2Δipod*plg+/+ (n=10) and nphs2Δipod*plg−/− mice (n=11) receiving vehicle and 93 ± 11 and 91 ± 11 μmol 6 h−1 receiving amiloride injection. In nephrotic nphs2Δipod*plg+/+ (n=9) and nphs2Δipod*plg−/− mice (n=8) vehicle injection was followed by a significantly reduced urinary sodium excretion (3 ± 1 and 5 ± 1 μmol 6 h−1, respectively). After amiloride injection urinary sodium excretion increased to 110 ± 26 and 159 ± 27 μmol 6 h−1, respectively.
(B) Amiloride-sensitive natriuresis as the fold increase. In uninduced nphs2Δipod*plg+/+ and nphs2Δipod*plg−/− mice this ratio was 4 ± 1 (n=10) and 9 ± 3 (n=11), respectively, in nephrotic nphs2Δipod*plg+/+ and nphs2Δipod*plg−/− mice 72 ± 26 and 63 ± 16, respectively.
(C, D) Course of urinary sodium excretion in spot urine samples and body weight taken in the morning after induction of nephrotic syndrome in nphs2Δipod*plg+/+, nphs2Δipod*plg−/− mice and control mice, respectively.
Arithmetic means ± SEM, # indicates significant difference to baseline value, * indicates significant difference between genotypes, § indicates significant difference between uninduced and nephrotic state. A: amiloride; U: uninduced; N: nephrotic. V: vehicle.