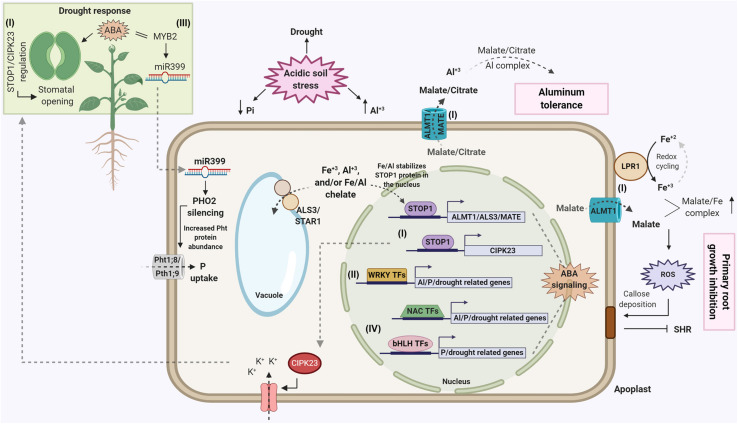
FIGURE 1.
Model for cross-talk between Al toxicity, low-P availability and drought stress. Four transcriptional regulatory networks are highlighted that we identified in this review that may be involved with plant responses to these three abiotic stresses. (I) The SENSITIVE TO PROTON RHIZOTOXICITY 1 (STOP1) transcription factor is involved in Al tolerance, P deficiency and drought stress. In addition to AtMATE, STOP1 transcriptionally activates the ALUMINUM ACTIVATED MALATE TRANSPORTER1 (ALMT1) which encodes the root PM anion channel that mediates Al activated malate release from roots, detoxifying Al in the rhizosphere. P deficiency induces the release of the multicopper ferroxidase (LPR1) that reduces/oxidizes Fe. P deficiency also induces expression of ALMT1, and the malate released via the ALMT1 protein chelates and facilitates accumulation of Fe in the cell wall where its oxidoreduction is catalyzed by LPR1, generating peroxides, which triggers lignification and cell wall stiffening, and rapid inhibition of root growth. At the same time, ROS generated from oxidoreduction of Fe triggers callose formation, which blocks plasmodesmata between stem cell initials and cells of the RAM outside the stem cell niche. This blockage of the plasmodesmata prevents cell-to-cell transport of the transcription factor, SHORT-ROOT, which is needed to drive stem cell division. This exhausts the RAM and terminates primary root growth. The Fe accumulated in the cell wall also drives increased Fe influx into the cytoplasm of RAM and the increased Fe helps stabilize and enhance STOP1 accumulation in the nucleus. Under P sufficient conditions, the tonoplast ABC transporter, ALS3/STAR1, is hypothesized to transport the Fe or Al (depending on soil conditions) or possibly Fe/Al-chelates into the vacuole and the reduction of Fe/Al levels in the cytoplasm and nucleus represses STOP1 protein accumulation in the nucleus, inhibiting ALMT1, transcriptional activation. With regards to STOP1’s involvement in drought response, STOP1 also transcriptionally activates CBL-INTERACTING PROTEIN KINASE 23 (CIPK23) expression, and the CIPK23 protein is an activator of high affinity K+ transporters in the root and possibly the shoot, which could result in enhanced stomatal opening and a reduction in drought tolerance due to increased transpirational water loss. (II) A second family of candidate TFs are the large WRKY family. There are several WRKY transcription factor family members involved in responses to drought and Al toxicity. For example, AtWRKY46 represses ALMT1 expression in the absence of Al and also is expressed in guard cells in response to drought stress; although its role in drought response remains to be elucidated (III) The AtMYB2 transcription factor co-regulates P efficiency and is involved in drought response via regulation of miR399. AtMYB2 induces miR399 in response to ABA and salt stress, and overexpression of miR399 results in salt and ABA tolerance but interestingly, is associated with drought sensitivity. miR399 plays a well-documented role in long distance P deficiency signaling in the phloem as it is synthesized in response to P deficiency in mature source leaves and is translocated in the phloem to the root, where it silences PHOSPHATE 2 (PHO2), which encodes a ubiquitin-conjugating E2 enzyme that negatively regulates P transporters under P sufficiency. (IV) NAC and Basic helix-loop-helix (bHLH) transcriptional factors have been implicated in drought regulation of stress-related genes via a wide-range of possible tolerance mechanisms, which involves ABA-dependent and independent pathways. The bHLH family member, PTF1, plays a role in low P tolerance, enhancing lateral root development. ZmPTF1 bind to the promoter of NAC30 and other TFs, acting as a positive regulator of those genes. Thus, a possible connection between NAC-mediated tolerance to both drought and low-P conditions may be mediated at some extent via bHLH-dependent regulation of NAC TFs. NAC TFs may also connect with Al tolerance based on up-regulation of VuNAR1 root apex and Al inducibility of other NACs. VuNAR1 also binds to the promoters of wall-associated receptor kinase genes, conferring Al tolerance through regulation of cell wall pectin metabolism. Although there is no evidence for direct interaction of STOP1, AtMATE1 and AtALS3 promoters, it is clear that STOP1 is crucial for induction of these genes. Not shown here is a possible link between Al tolerance and drought tolerance via ERF transcription factors, whose supporting evidence is more limited and preliminary. The figure was created with BioRender.com.