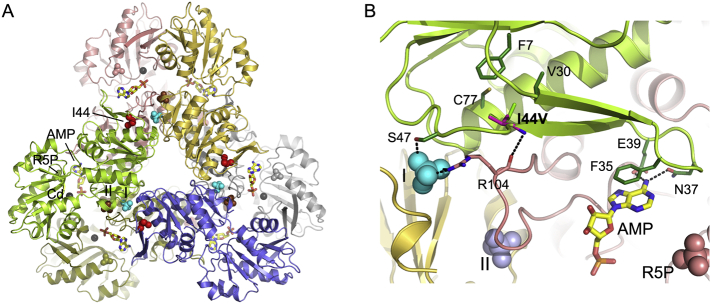
Fig. 2.
PRPS-1 structure and Ile44Val substitution site. (A) The crystal structure of PRPS-1 (PDB entry 2HCR), with each subunit colored differently. AMP (stick model colored by atom) and a Cd2+ ion (grey sphere) indicate the location of the ATP-Mg2+ binding site in the interface between the N- and C-terminal domain; the binding site for the second substrate R5P is occupied by a sulfate ion shown as a space-fill model colored as the corresponding subunit. In the crystal structure, allosteric sites I and II are also occupied by sulfate ions, shown as space-fill models in brown and cyan, respectively. The mutation site is indicated by a space-fill model of the Ile-44 side chain in red. The respective binding sites are labeled only for the green subunit. (B) Close-up view of the Ile44Val substitution site. For the valine (shown as sticks with carbon atoms in magenta), the same side chain conformer as adopted by Ile-44 (sticks with carbon atoms in green) in the crystal structure is chosen. Subunits and ligands are depicted as in (A). Residues interacting with Ile-44 or involved in ligand binding that are mentioned in the text are shown as sticks with carbon atoms in the same color as the subunit to which they belong. Hydrogen bonds are indicated as black dotted lines.