Abstract
Background
Consolation is a type of empathy-like behavior that has recently been observed in some socially living rodents. Despite the growing body of literature suggesting that stress affects empathy, the relationship between stress and consolation remains understudied at the preclinical level. Here, we examined the effects of chronic emotional stress or physical stress exposure on consolation and emotional behaviors by using the socially monogamous mandarin vole (Microtus mandarinus) in both males and females.
Method/Results
Physical stress voles were exposed to 14-day social defeat stress, whereas emotional stress voles vicariously experienced the defeat of their partners. We found that physical stress, but not emotional stress, voles showed reduced grooming toward their defeated partners and increased anxiety- and despair-like behaviors. Meanwhile, physical stress voles exhibited decreased neural activity in the anterior cingulate cortex, which is centrally involved in empathy. The densities of oxytocin receptors, dopamine D2 receptors, and serotonin 1A-receptors within the anterior cingulate cortex were significantly decreased in the physical stress group compared with controls. All the behavioral and physiological changes were similar between the sexes. Finally, we found that the reduced consolation behavior and some anxiety-like syndromes in physical stress voles could be alleviated by pretreatment with an oxytocin receptor, D2 receptors, or serotonin 1A-receptor agonist within the anterior cingulate cortex, whereas injections of corresponding receptor antagonists to the control voles decreased the consolation behavior and increased some anxiety-like behaviors.
Conclusions
Our results indicated that chronic physical stress exposure impaired consolation and induced anxiety-like behaviors in mandarin voles and oxytocin receptors, 5-HT1A receptors, and D2 receptors within the anterior cingulate cortex may play important roles in these processes.
Keywords: consolation, social defeat, anterior cingulate cortex, Mandarin voles
Significance Statement.
Consolation is a type of empathy-like behavior that has recently been observed in some socially living animals. Although studies indicate that stress affects empathy, the relationship between stress and consolation remains understudied at the preclinical level. In this study, by using the socially monogamous mandarin vole, we found that a 14-day social defeat stress reduced allogrooming (an indicator of consolation behavior) towards a distressed partner and induced some anxiety- or depression-like behaviors. Along with these behavior changes, the expressions of OTRs, dopamine D2Rs, and 5-HT1ARs within the ACC were significantly downregulated. Pharmacological studies indicated that the decreased OTR, D2R, and 5-HT1AR activities may be involved in the behavioral changes in the defeated individuals. Our study is meaningful as we provide a basis for further examination of the impaired empathy that is frequently observed in many mood-related illnesses, such as depression, autism, and schizophrenia.
Introduction
Stress is a ubiquitous life experience that influences the daily behaviors and social functions of organisms. Many factors influence the pattern and magnitude of the stress response, for example, the type of stressor, the developmental stage of the animal, and the animal’s sex and genetic background (Joëls and Baram, 2009). Empathy refers to sharing or relating to the affective states of another, which could be divided into 3 levels according to de Waall’s definition (“emotional contagion,” “consolation,” and “perspective taking and targeted helping”) (de Waal and Preston, 2017). Consolation, defined as an increase in affiliative contact directed toward a distressed individual by an uninvolved bystander, has long been assumed to exist in species possessing complex cognitive functions, such as humans, apes, dolphins, and elephants (Pérez-Manrique and Gomila, 2018). However, recent studies have indicated that this behavior can also be elicited in rodents (Burkett et al., 2016; Li et al., 2019). Impaired consolation has been frequently observed in depressed patients. For example, depressed mothers usually have less concern for the crying of their newborn babies (Field et al., 2009) and engage in less affective touching of their babies than healthy mothers did (Young et al., 2015). However, preclinical studies seeking a deep understanding of the causal relationship between consolation and depression have lagged considerably behind, partly due to a lack of suitable animal models.
The chronic social defeat stress (CSDS) paradigm has been one of the most robust models widely used to study the neurobiology of depression and other stress-related illnesses, given its high ethological and pharmacological validity (Hammels et al., 2015). However, this model has proven difficult to implement in most female rodents because they are less territorial than males (Hammels et al., 2015). This is problematic because depression and anxiety disorders are more common in women than men and because some depressive symptoms are sexually dimorphic (Dean and Keshavan, 2017). The mandarin vole (Microtus mandarinus) is a socially monogamous rodent that is widely distributed across China. Both adult male and female mandarin voles show strong spontaneous territorial defense behavior (Tai and Wang, 2001). In our previous study, we found that consolation behavior could be elicited in mandarin voles by exposure to a defeated partner (Li et al., 2019). Therefore, it is an ideal animal model to investigate the relationship between consolation and stress as well as any potential sex differences.
A growing body of literature suggests that emotional and/or psychological stress (ES) may also play a critical role in the etiology of mood-related psychopathology. Both human and animal studies have indicated that depression-like behaviors can be induced vicariously in individuals who only witness a traumatic event or even those who are merely exposed to a distressed partner (Karp, 2017; Iñiguez et al., 2018). However, the CSDS model is unable to tease apart the different effects of emotional vs physical stress (PS), since the defeated subjects are exposed to both stressors as currently performed. Iniguez et al. provided an elegant ES model in which the subjects witness the defeat of the intruders, after which the “observers” exhibit depressive-like behaviors similar to those of the defeated animals (Warren et al., 2013; Iñiguez et al., 2018). On this basis, we plan to investigate the effects of both chronic PS and ES exposure on consolation behaviors in the present study.
Efforts have been made to examine the underlying neural mechanisms of empathy-like behavior. One molecular substrate is oxytocin (OT). In humans, intranasal OT is believed to suppress stress and enhance empathy (Hurlemann et al., 2010). Similarly, Zoratto et al. found that OT administration promotes emotional contagion in mice (Zoratto et al., 2018). OT produces physiological effects mainly through its only receptor, the OTR. It has been demonstrated that OTR activation within the anterior cingulate cortex (ACC) is necessary for consolation behavior (Burkett et al., 2016; Li et al., 2019). In addition, the closely related arginine vasopressin (AVP) is also known to play important roles in a variety of social behaviors, stress adaptation, and anxiety (Neumann and Landgraf, 2012), and there is evidence that some behavioral effects of OT work through the vasopressin V1a receptor (V1aR) (Ramos et al., 2013). Although our previous studies indicated that the V1aR may not be involved in consolation under normal circumstances (Li et al., 2019), whether the situation would be the same in a depressed state has yet to be examined.
The other candidate neurotransmitter systems are the serotonin (5-HT) and dopamine (DA). It is well known that 5-HT regulates mood, behavior, and numerous physiological functions (Berger et al., 2009). There are at least 16 different serotonin receptors in the brain, of which the 5-HT1A receptors (5-HT1AR) are among the best characterized (Artigas, 2013). Kim et al. investigated the relationship between 5-HT and empathy-like behavior in mice (Kim et al., 2014). They found that microinjection of 5-HT into the ACC impaired vicarious fear and altered the regularity of neural oscillations. However, administration of methysergide (a 5-HT receptor antagonist) had no effect on observational fear learning. The DA system is another key modulator of emotion and behavior. DA receptors are widely distributed in the CNS, and the most abundant subtypes are D1R and D2R (Pytka et al., 2016). The abovementioned study by Kim et al. found that D2Rs but not D1Rs in the ACC are required for vicarious fear (Kim et al., 2014). However, microinjection of DA into the ACC had no apparent effect on this empathy-like behavior. Overall, the functional roles of 5-HT and DA systems in empathy-like behavior still require further exploration.
The present study was conducted to address these unanswered questions by examining (1) the effects of chronic PS and ES exposure on consolation and emotional behaviors in both male and female mandarin voles; (2) the expression profiles of OTR, V1aR, 5-HT1AR, D1R, and D2R upon PS and ES exposure; and (3) pharmacological evidence validating specific receptors’ function in the decrease in consolation on PS exposure. As evidence indicates that the ACC plays essential roles in empathy-like behaviors (Meyza et al., 2017), we focused our studies on this subregion of the medial prefrontal cortex (mPFC, which contains the ACC, prelimbic cortex [PrL], and infralimbic cortex [IL] subregions in rodents).
Materials and Methods
Animals
The mandarin voles used in this experiment were laboratory-bred animals whose ancestors were derived from a wild population from Lingbao (Henan, China). The voles were weaned on postnatal day 21 and socially housed in polycarbonate cages (44 × 22 × 16 cm). Subjects were maintained on a 12-h-light/12-h-dark light photoperiod with unlimited access to carrots and rabbit chow. The voles used in this study were about 70 days old at the time. All breeding, housing, and experimental procedures were in accordance with Chinese and NIH guidelines for the care and use of laboratory animals and were approved by the Animal Care and Use Committee of Shaanxi Normal University.
Study Procedures and Grouping
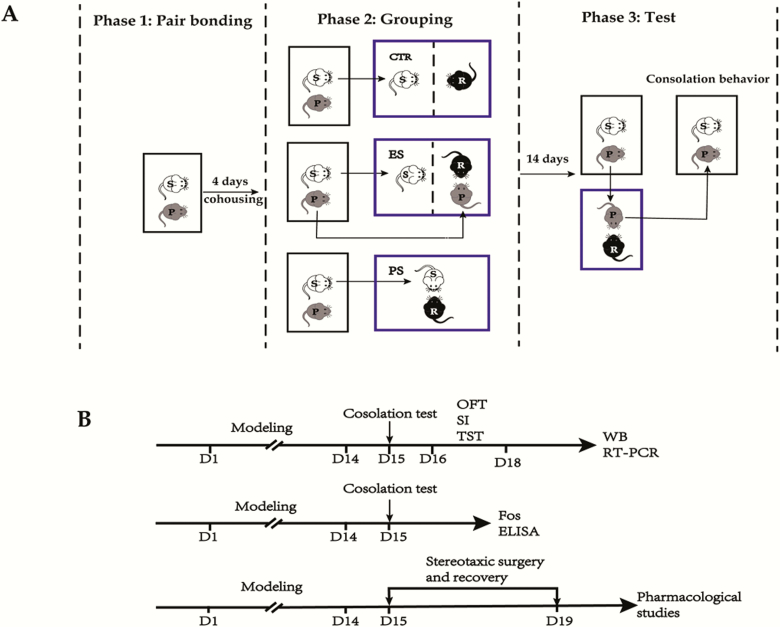
The study procedures and the timeline are depicted in Figure 1. Unless stated otherwise, all behavioral studies were conducted under dim light during the dark phase of the light-dark cycle (approximately 8:00–10:00 pm).
Figure 1.
Schematic of the study procedure (A) and timeline of experimental design (B). Firstly, the voles were cohoused for 4 days to allow for the formation of a pair bond (phase 1). Then the subjects were randomly assigned to 1 of 3 groups: control (CTR), emotional stress (ES), and physical stress (PS) (phase 2). In the CTR group, the subjects were put in the residents’ cage where they were separated from each other by clear perforated acrylic glass. In the ES group, the subjects were put in cages where they would witness the social defeat bouts of their partners by an aggressive resident. In the PS group, the subjects were directly put in the residents’ cage without separators where they were defeated by the resident. Following each session (10 min/d), the subjects were returned to their original home-cages and cohoused with their partners. This procedure lasted for 14 consecutive days and then the subject voles were tested on the consolation test (phase 3), where their partners were defeated and the consolation behaviors of the subjects were recorded. For detailed procedures, please refer to the text. OFT, open-field test; P, partners; R, residents; S, subjects; SI, social interaction test; TST, tail suspension test; WB, western blot.
Initially, adult male and female voles were cohoused for 4 days to allow for the formation of a pair bond (Yu et al., 2012). Thereafter, the subjects (marked by cutting a bundle of hair on the back) were randomly assigned to 1 of 3 groups: PS, ES, and control. The PS was based on a classical “resident-intruder” paradigm with a little modification (Wang et al., 2019). On the experimental day (day 1), subjects (intruder) were removed from their cages and placed for 10 minutes in the cage of an aggressive same-sex resident vole. Sooner or later, the subjects were attacked and defeated by the resident. At the end of this 10-minute period, the subjects were reunited with their respective partners, who were otherwise left undisturbed in their home cages during the social defeat procedure. This procedure was repeated for 14 consecutive days (days 1–14) at the onset of the dark phase of the light/dark cycle. Body weight fluctuations of the subjects were monitored for every 2 days from day 1. To avoid large individual differences in the intensity of received aggression, we exposed the subjects to a different resident vole every time in a rotational design.
We define ES as nonphysically experiencing defeat stress. The study protocol was adapted from Iniguez’s study (Iñiguez et al., 2018). Briefly, the subjects were placed in the residents’ cages, but they were separated from each other by clear perforated acrylic separators. Then, the subjects’ partners as intruders were placed into the same compartment as the residents. The subjects then vicariously experienced the defeat of their partners by the aggressive residents. Following each session (10 min/d), both the subjects and their partners were returned to their original home cages. To rule out any confounding effects related to activity or olfactory stimuli originating from the aggressive residents, the control subjects were also placed in the residents’ cages but separated from each other by clear perforated acrylic glass separators.
Consolation Test
Twenty-four hours after the last stress episode (day 15), all groups (n = 11 in each group for each sex) of subjects were tested for consolation behaviors as in our previous study (Li et al., 2019), with slight modification. This time, all the subjects’ partners were defeated for 10 minutes by an aggressive resident, and the subjects were otherwise left undisturbed in their home cages. When the 10-minute period had passed, the pairs were reunited, and the behavior of the subjects was recorded using a video camera for 10 minutes in the test room. The digital videos were viewed and quantified using J Watcher software (http://www.jwatcher.ucla.edu/) by raters who were blinded to the experimental groups and treatments. Videos were coded for allogrooming as indicators of consolation (Burkett et al., 2016; Li et al., 2019). Allogrooming was defined as head contact with the body or head of another individual, accompanied by a rhythmic head movement. Grooming directed toward the genitals, anogenital region, or tail was classified as sexual grooming and excluded.
Anxiety- and Despair-Like Behavior Assay
Following the consolation test, all the subjects (n = 10–11 in each group for each sex) were tested with a battery of mood-related tasks, that is, the open-field test (OFT), the social interaction test (SI), and the tail suspension test (TST). One day after the consolation test (day 16), the 5-minute OFT was conducted, followed by the two 5-minute phases of the SI. We used this sequence to eliminate the potential effect of stimulus voles in the SI on a subsequent OFT if the test sequence were reversed. The TST was performed 2 days after the consolation test (day 17). The detailed procedures of these behavioral tests are presented in the Supplementary methods.
Quantitative RT-PCR and Western Blot
Quantitative reverse transcription-polymerase chain reaction (RT-PCR) and Western blot (WB) were used to measure both mRNA and protein expression profiles of OTR, V1AR, D1R, D2R, and 5-HT1AR among the groups (n = 5−6 in each group for each sex). The primer sequences are presented in supplementary Table 1. For details, please refer to the supplementary Methods.
Immunofluorescence
To investigate neuronal activity differences within the ACC along with the consolation behavior, c-Fos expression profiles were examined 90 minutes after the consolation test. The detailed procedures are presented in the supplementary Methods.
CORT Assay
The plasma collection procedures are presented in the supplementary Methods. The levels of corticosterone (CORT) in the plasma (n = 6 in each group for each sex) were quantified with a commercially available ELISA kit (H205, Nanjing Jiancheng Biotechnology, China) according to the manufacturer’s instructions. All samples were performed in duplicate. The intra- and inter-assay coefficients of variation were 8.5% and 10%, respectively.
Pharmacological Studies
To test whether the reduced expression of OTR, D2R, and 5-HT1AR in the ACC are involved in the behavior changes in the PS voles, we first investigated the effects of microinjections of OTR, D2R, and 5-HT1AR agonists into the ACC of the PS voles. On the other hand, both the agonists and respective antagonists were administrated to the nonstressed control voles to eliminate the general effect of these drugs and test the necessity of decreased receptor activity. Five cohorts of animals were used in this experiment, and each cohort had a separate saline control group. The stereotaxic cannulation surgery procedures are presented in the supplementary Methods.
For microinjection and behavior assay, on each testing day, the animals were given a bilateral injection of vehicle (saline, 2 μL/side), OT (1, 10, 100 ng/0.2 μL per side; BACHEM, Bubendorf, Switzerland), 8-OH-DPAT (5-HT1AR agonist; 0.03, 0.3, 3 μg/0.2 μL per side; Sigma) or quinpirole (D2R agonist; 1, 10, 100 ng/0.2 μL per side; Sigma) to the PS-voles. On the other hand, OT (10 ng/0.2 μL per side), 8-OH-DPAT (3 μg/0.2 μL per side), quinpirole (10 ng/0.2 μL per sid), the oxytocin receptor antagonist (OTA; 50 ng/0.2 μL per side; d(CH2)5,Tyr(Me)2,Thr4,Tyr-NH29-OVT; BACHEM, Bubendorf, Switzerland), WAY-100635 (the 5-HT1AR antagonist; 0.4 μg/0.2 μL per side; Sigma) or raclopride (D2R antagonist; 0.5 μg/0.2 μL per side; Sigma) were administrated to the normal control voles. The speed of injection was 0.1 μL/min. The injector tips remained in situ for another 2 minutes for drug diffusions. About 20 minutes had passed before the first consolation test. Subsequently, the OFT and the SI were conducted to determine whether anxiety-like behavior was affected by the drugs. All the behavior studies were finished within 40 minutes after the injections. The dose and timing of drug administration were chosen based on our preliminary study and also the following previous studies: OT and OTA (Burkett et al., 2016; Dong et al., 2017), 8-OH-DPAT and WAY-100635 (Cooper et al., 2008; Wang et al., 2019), and quinpirole and raclopride (Liu and Wang, 2003; Watt et al., 2014).
Finally, cannula placement was confirmed through histological localization of the guide cannula on slide-mounted brain sections. Only subjects with correct cannula placements and normal motor abilities were included in the final data analysis (n = 5–6 in each group for each sex).
Data Analyses
In general, all females were autopsied for pregnancy after all the experiments. If pregnant, the whole data were removed from the following analysis (most mandarin vole give their first birth at 4 months of age [observation data]; the pregnancy rate was about 10% in this study). Unless explicitly stated, 2-way ANOVA was the default analysis method, with sex (male, female) and treatment (control, ES, PS) as between-subjects factors. Body weight data were analyzed by repeated measures, with time (days), treatment, and sex as between-subjects factors, and sphericity assumptions were checked by Mauchly’s test. If no effects of sex or sex and other factor interactions were found, data from both sexes were combined for further analysis and plotting. Bonferroni tests were used for post-hoc comparisons. All the statistical procedures were performed using SPSS 20.0. Data are presented as mean ± SE unless otherwise indicated and the significance level was set at P < .05 (2-tailed).
Results
PS Exposure Reduces Consolation Behaviors
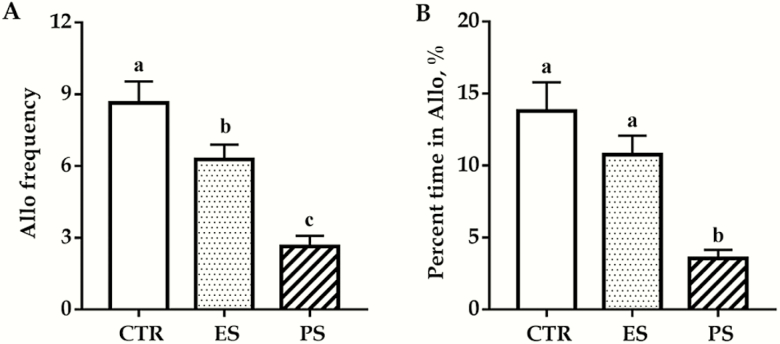
The effects of PS and ES exposure on consolation behaviors are shown in Figure 2. Our data showed a significant main effect of treatment on both the frequency of and the time spent on allogrooming (F(2,60) = 20.0 and 13.3, respectively; all P < .01). PS voles displayed a significantly lower frequency and less time on allogrooming than both control and ES voles (all P < .01; Figure 2A–B). ES voles showed a reduced allogrooming frequency than controls (P < .05; Figure 2A) but not in total allogrooming time (P = .44; Figure 2B). No overall sex and sex × treatment interaction differences were seen for both the frequency of and the time spent on allogrooming (F(1,60) = 1.0, 0.3, P = .32, 0.59 for sex; F(2,60) = 1.6, 0.4, P = .22, 0.65 for sex × treatment interaction).
Figure 2.
Effects of physical stress (PS) or emotional stress (ES) exposure on frequencies (A) and time spent (B) in allogrooming. Data are presented as mean ± SE, n = 22 in each group (11 males, 11 females); groups not sharing the same letter significantly differ from each other. Allo, allogrooming; CTR, control.
PS Increases Anxiety- and Despair-Like Behaviors
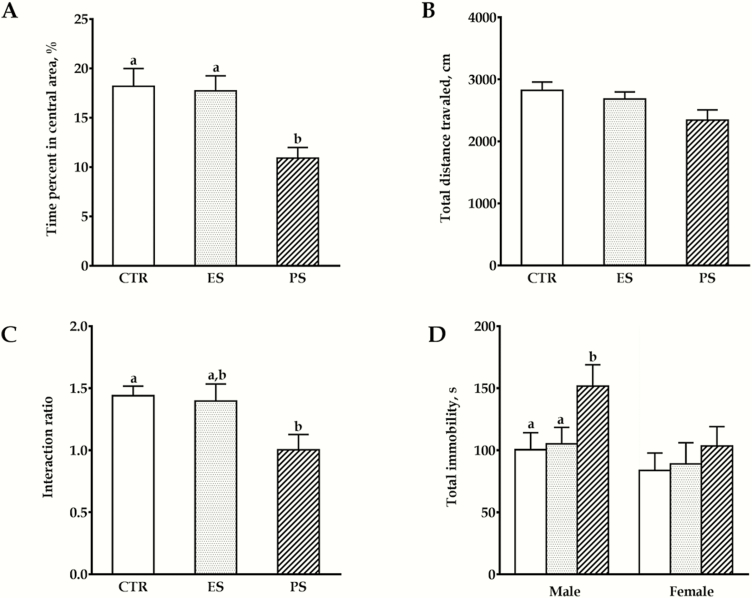
The effects of PS and ES exposure on anxiety- and despair-like behaviors are shown in Figure 3. In the OFT, our data showed that there was a significant main effect of treatment on the time spent in the central zones (F(2,60) = 10.1, P < .01). PS voles spent significantly less time in the central area than both controls and ES voles, which indicates an increased anxiety level in a novel environment (all P < .01; Figure 3A). No sex and sex × treatment interaction differences were seen (F(1,60) = 0.5, P = .47; F(2,60) = 1.0, P = .36; respectively). For the total distance traveled, no overall sex (F(1,60) = 0.06, P = .80), treatment (F(2,60) = 3.1, P = .06), or their interaction differences were seen (F(2,60) = 1.3, P = .29; Figure 3B).
Figure 3.
Effects of physical stress (PS) or emotional stress (ES) exposure on anxiety- and despair-like behaviors. Time in the central area (A) and total distance moved (B) in the open field test (OFT). (C) Social interaction ratios in the social interaction test (SI). (D) Total immobility time in the tail suspension test (TST). Data are presented as mean ± SE; n = 22 for graph A‒C (11 males, 11 females), n = 10 for graph D in each for each sex. Groups not sharing the same letter significantly differ from each other. CTR, control.
In the SI, there was also a significant main effect of treatment on the social interaction ratio (F(2,60) = 7.0; P < .01). PS voles displayed a significantly lower social interaction ratio compared with controls, which indicated a lowered sociability (Figure 3C; P < .01). Similarly, no overall sex and sex × treatment interaction differences were seen (F(1,60) = 0.2, P = .65; F(2,60) = 0.3, P = .74; respectively).
In the TST, our data showed that there was a significant main effect of treatment (F(2, 54) = 20.2, P < .01) and a sex × treatment interaction (F(2, 54) = 3.6, P < .05). Specifically, compared with controls, PS males spent significantly more time in the immobile position (Figure 3D; P < .05), which may indicate more sensitivity to this inescapable stress mode (despair). However, such effects were not found in females (P = .08, PS vs control).
Body Weight Variations
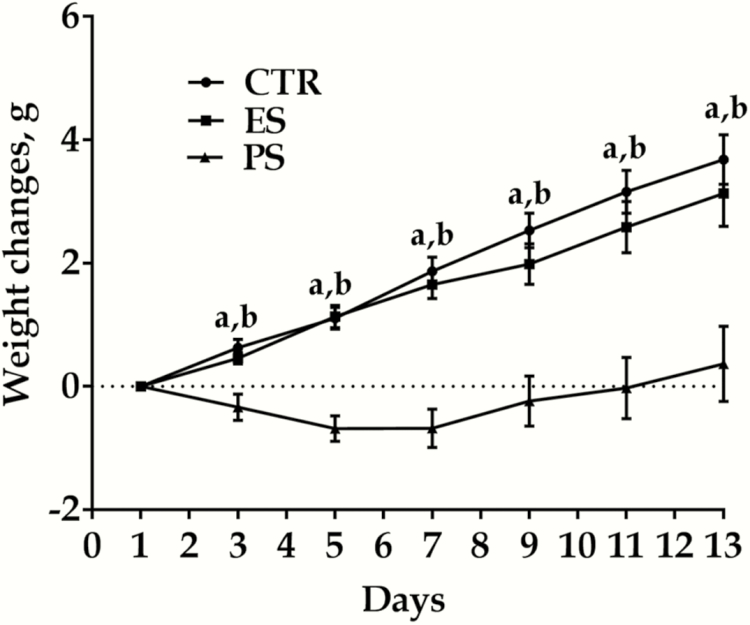
Figure 4 shows body weight changes among the groups. Body weight changes were calculated by subtracting the recorded weight of the animal from the weight on the initial day of the experiment; thus, a positive number would indicate an increase in overall body weight, and a negative number would indicate body weight decrease. Three-factor repeated-measures ANOVA indicated weight changed as a function of treatment (F(2,42) = 27.9, P < .01), time (F(6,126) = 61.7, P < .01), and their interaction (F(12, 252)= 9.8, P < .01). PS voles especially displayed significantly lower body weight compared with both controls and ES groups from day 3 to 13. There were no significant different for PS vs ES groups for all time points (day 2‒13: P = .455, 0.578, 0.155, 0.184, 0.159, and 0.148, respectively).
Figure 4.
Effects of physical stress (PS) or emotional stress (ES) exposure on body weight variations. Data are presented as mean ± SE, n = 22 in each group (11 males, 11 females); aP < .01 for control vs PS comparisons. bP < .05 for ES vs PS comparisons. CTR, control.
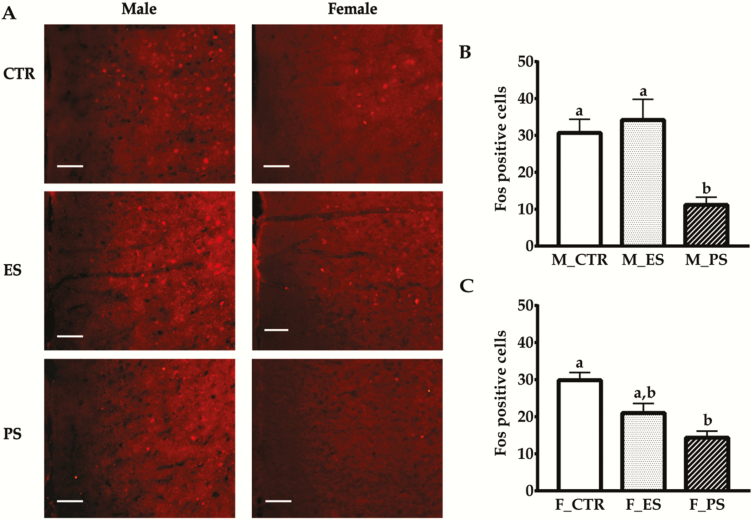
PS Exposure Reduces c-Fos Expressions in the ACC
The effects of PS and ES exposure on c-Fos expressions in the ACC are shown in Figure 5. Our data showed there was a significant main effect of treatment (F(2, 30) = 16.6; P < .01) and a sex × treatment interaction (F(2, 30) = 3.5; P < .05). In males, Fos-positive cells were significantly reduced in PS groups compared with the other 2 groups (all P < .01), which, in turn, did not differ from each other (P = 1.0; Figure 5B). In females, the difference was only found in PS vs control comparison (P < .01; Figure 5C).
Figure 5.
Effects of physical stress (PS) or emotional stress (ES) exposure on c-Fos expressions in the anterior cingulate cortex (ACC). (A) Representative images of Fos-ir positive cells for all of the groups. (B–C) Fos-ir positive cells quantifications in male and female groups, respectively. Data are presented as mean ± SE, n = 6 in each group; groups not sharing the same letter significantly differ from each other. CTR, control; F, female; M, male. Bar = 200 μm.
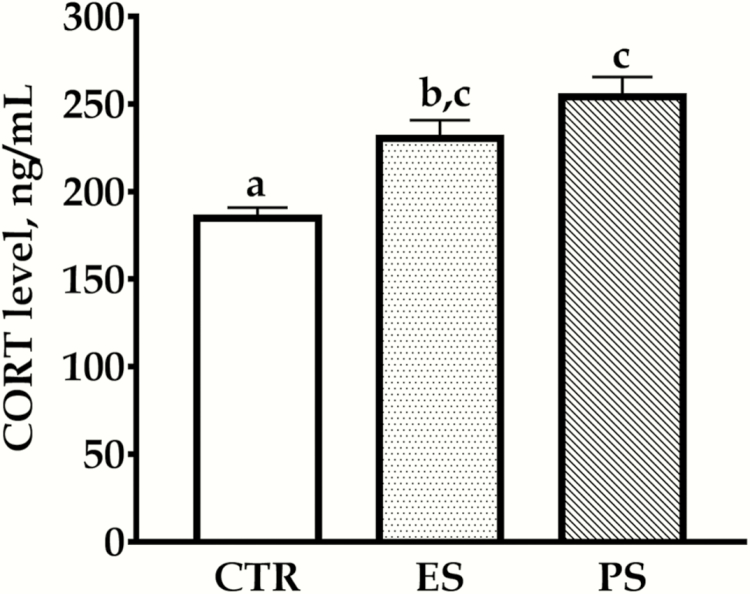
Plasma CORT Levels
The ELISA test results are depicted in Figure 6. The data showed a significant main effect of treatment on plasma CORT levels (F(2, 30) = 12.5; P < .01) but no effect of sex and their interactions (F(1,30) = 0.09, P = .76; F(2,30) = 0.1, P = .90, respectively). Plasma CORT levels were significantly higher in both PS and ES voles compared with control groups (Figure 6, all P < .01). There was no significant difference for ES vs PS (P = .31).
Figure 6.
Effects of physical stress (PS) or emotional stress (ES) exposure on plasma corticosterone (CORT) levels. Data are presented as mean ± SE, n = 12 in each group (6 males, 6 females); groups not sharing the same letter significantly differ from each other. CTR, control.
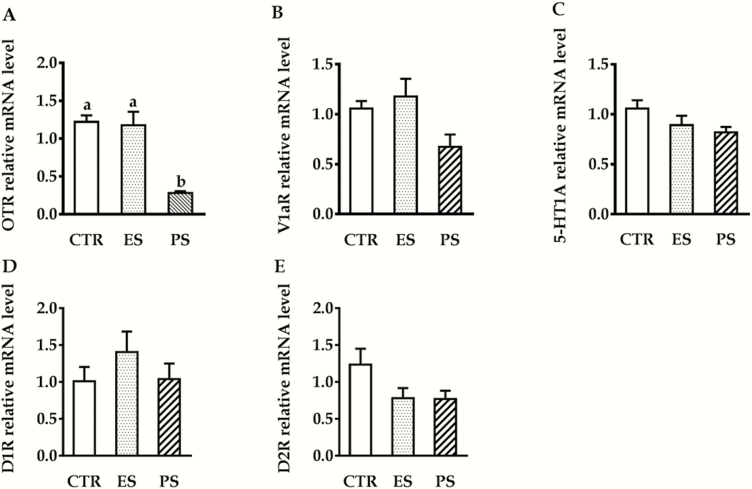
RT-PCR Results
Figure 7 shows the RT-PCR results of OTR, V1AR, D1R, D2R, and 5-HT1AR mRNA expression in the ACC. Our data showed that there was a significant main effect of treatment on the OTR mRNA expression (F(2,24) = 19.4; P < .01). Follow-up analysis indicated PS voles displayed a significantly lower OTR mRNA expression compared with both control and ES voles (Figure 7A, all P < .01). No overall sex, treatment, or their interactions differences were seen in V1AR (F(1,24) = 0.6, P = .22; F(2, 24) = 2.0, P = .15; F(2,24) = 1.1, P = .35, respectively), 5-HT1AR (F(1,24) = 0.01, P = .98; F(2,24) = 2.2, P = .13; F(2,24) = 0.08, P = .92, respectively), D1R (F(1,24) = 0.8, P = .38; F(2,24) = 0.98, P = .39; F(2,24) = 2.1, P = .14, respectively), and D2R mRNA (F(1,24) = 0.06, P = .8; F(2,24) = 2.4, P = .11; F(2,24) = 0.04, P = .96, respectively) expression (Figure 7B–D).
Figure 7.
Effects of physical stress (PS) or emotional stress (ES) exposure on oxytocin receptor (OTR) (A), vasopressin V1a receptor (V1AR) (B), serotonin 1A receptor (5-HT1AR) (C), dopamine D1 subtype receptor (D1R) (D), and dopamine D2 subtype receptor(D2R) (E) mRNA expression in the anterior cingulate cortex (ACC). n = 10 in each group (5 males, 5 females); groups not sharing a same letter are significantly different from each other. CTR, control.
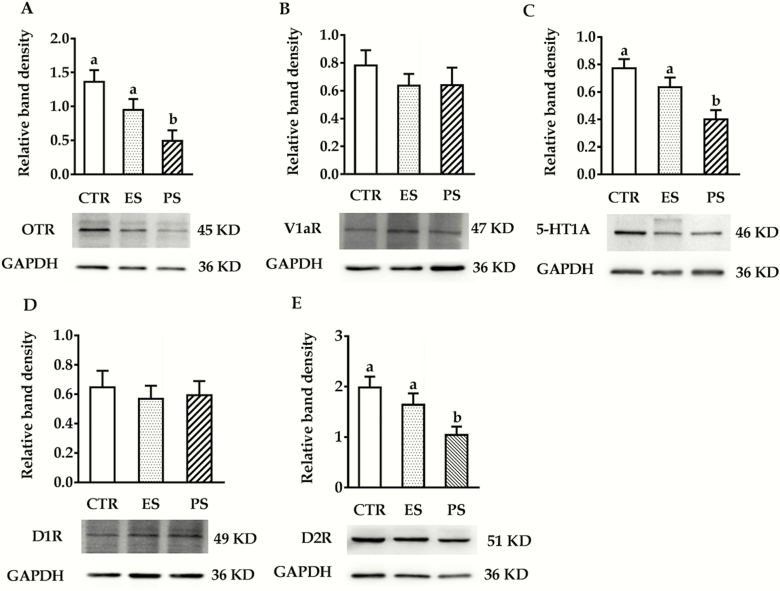
WB Analysis
WB results of OTR, V1AR, 5-HT1AR, D1R, and D2R expression in the ACC are shown in Figure 8 (the untruncated images were shown in supplementary Figure 1). In terms of OTR, 5-HT1AR, and D2R expression, we found a significant main effect of treatment (F(2,30) = 7.0, 7.2 and 5.6, respectively, all P < .01) but no sex (F(1,30) = 2.3, P = .14; F(1,30) = 0.4, P = .53; F(1,30) = 4.0, P = .054, respectively) or sex × treatment interaction (F(2,30) = 0.09, P = .91; F(2,30) = 0.87, P = .43; F(2,30) = 0.94, P = .40, respectively). Post-hoc analysis revealed significantly lower OTR, 5-HT1AR, and D2R expressions in the PS groups compared with controls (all P < .05). There was no difference for ES vs control comparisons (P = .25, 0.52, 0.43, respectively; Figure 8A,C and E). With regards to V1AR and D1R expressions, no overall sex (F(1,30) = 3.4, P = .08; F(1,30) = 2.2, P = .15, respectively), treatment (F(2,30) = 0.6, P = .54; F(2,30) = 0.15, P = .86, respectively) or their interactions (F(2,30) = 0.71, P = .50; F(2,30) = 0.71, P = .50; F(2,30) = 0.02, P = .98, respectively) differences were found (Figure 8B,D).
Figure 8.
Effects of physical stress (PS) or emotional stress (ES) exposure on oxytocin receptor (OTR) (A), vasopressin V1a receptor (V1AR) (B), serotonin 1A receptor (5-HT1AR) (C), dopamine D1 subtype receptor (D1R) (D), and dopamine D2 subtype receptor(D2R) (E) densities in the anterior cingulate cortex (ACC). n = 12 in each group (6 males, 6 females); groups not sharing the same letter significantly differ from each other. CTR, control.
Pharmacological Results
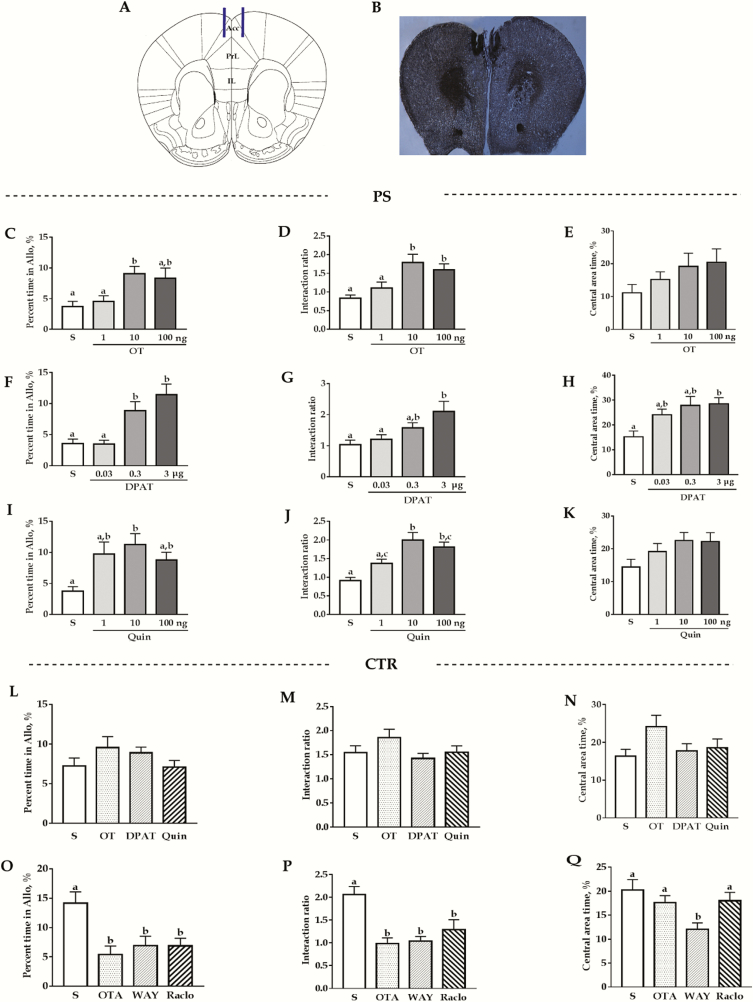
The pharmacological results are summarized in Figure 9. For all the drugs, no overall sex and sex × treatment interaction differences were seen (the detailed F and P values are presented in supplemental Table 2). In the consolation test, ANOVA results revealed that OT, 8-OH-DPAT, and quinpirole significantly affected the allogrooming time of PS voles (F(3,39) = 5.6, F(3,39) = 9.8, F(3,38) = 5.4, respectively; all P < .01). For OT, medium dose (10 ng) significantly increased the allogrooming time of PS voles (P < .01 vs saline), whereas low (1 ng) and high dose (100 ng) had no such effect (vs saline: P = 1.0 and 0.06, respectively; Figure 9C). For 8-OH-DPAT, both medium and high dose had such effect (0.3 μg and 3 μg vs saline; P < .05 and P < .01, respectively), but not at low dose (P = 1.0 vs saline; Figure 9F). For quinpirole, such effects were observed at medium dose but not at low and high dose (vs saline: P = .46 and 0.08, respectively; Figure 9I). The selected dosages of respective agonists had no significant effect (F(3,32) = 1.3; P = .28; Figure 9L), whereas the antagonist significantly attenuated the allogrooming time in control voles (F(3,34) = 5.4; P < .01, all P < .05 vs saline; Figure 9O).
Figure 9.
Effects of microinjection of different drugs into the anterior cingulate cortex (ACC) on the time spent in allogrooming of the consolation test, social interaction ratios in the social interaction test (SI), and time spent in the central area in the open-field test (OFT). (A) Schematic representation of ACC infusion sites. (B) A representative photomicrograph of the injection site (×20). (C–E) Effects of saline and different doses of oxytocin (OT). (F–H) Effects of saline and different doses of 8-OH-DPAT (DAPT). (I–K) Effects of saline and different doses of quinpirole (Quin) in PS voles. (L–N) Effect of saline, OT (10 ng/side), DAPT (3 μg/side), and raclopride (10 ng/side). (O–Q) Effect of saline, OT receptor antagonist (OTA) (50 ng/side), WAY-100635 (WAY) (0.3 μg/side), and raclopride (Raclo) (0.5 μg/side) on control subjects. Groups not sharing the same letter significantly differ from each other (P ≤ .05). Data are presented as the means ± SEM, n = 10–12 (5–6 males) in each group. Allo, allogrooming; CTR, control; TST, tail suspension test.
In the SI, we found OT, 8-OH-DPAT, and quinpirole significantly affected the interaction ratio of PS voles (F(3,39) = 7.2, P < .01; F(3,39) = 5.4, P < .01; F(3,38) = 10.9, P < .01). In detail, 10 ng and 100 ng OT significantly increased the interaction ratio (P < .01 and P < .05 vs saline, respectively) but not at 1-ng dosages (P = 1.0 vs saline; Figure 9D); for 8-OH-DPAT, 3 μg had such effect (P < .01 vs saline; Figure 9G), but not at 0.03 and 0.3 μg (vs saline: P = 1.0 and 0.52, respectively). For quinpirole, such effects were observed at 10- and 100-ng dosages (all P < .01 vs saline; Figure 9J). For the control voles, as in the consolation test, we found the selected dosages of OT, 8-OH-DPAT, and quinpirole had no significant effect (F(3,32) = 1.7, P = .19; Figure 9M), but OTA, WAY-100635, and raclopride treatments significantly decreased the social interaction ratio (F(3,34) = 8.6, P < .01; OTA and WAY-100635 vs saline, P < .01; raclopride vs saline, P < .05; Figure 9P).
In the OFT, ANOVA results revealed that 8-OH-DPAT significantly affected the time spent in the central zones of PS voles (F(3,39) = 4.5, P < .01), but no such effects were found in OT and quinpirole treatments (F(3,39) = 0.6, P = .12; F(3,38) = 2.3, P = .09; respectively). Further analysis revealed high (0.3 μg) dose of 8-OH-DPAT administration significantly increased the time spent in the central area (P < .01 vs saline; Figure 9H). In control voles, only WAY-100635 treatment significantly decreased the time spent in the central area (P < .05 vs saline; Figure 9N).
For all of the drugs, all the selected dosages had no significantly effect on the total distance traveled in the 5-minute OFT (supplemental Figure 2). The detailed P and F values are presented in supplemental Table 3.
Discussion
In this study, we attempted to investigate the effect of chronic PS and ES exposure on consolation behavior and the underlying neural mechanisms of reduced consolation on chronic PS exposure in mandarin voles. Using a battery of tests, we found that (1) chronic PS but not ES individuals showed reduced consolation towards a distressed partner and exhibited a series of depression-like syndromes; (2) compared with control, c-Fos expression within the ACC was significantly reduced in the PS group; (3) chronic PS exposure significantly reduced OTR, D2R, and 5-HT1AR protein expressions within the ACC; and (4) pharmacological results showed that the decreased OTR, D2R, and 5-HT1AR activities may be involved in the behavioral changes in the PS-exposed voles.
PS and ES
Just as in previous studies (Tse et al., 2019; Wang et al., 2019), we found that PS-defeated animals show a range of depression-like symptoms such as weight fluctuation, social aversion, anxiety, despair, and dysregulated hypothalamic-pituitary-adrenal (HPA) axis. Our novel findings are that PS-exposed voles displayed reduced grooming towards their defeated partners compared with controls. According to previous studies (Burkett et al., 2016; Li et al., 2019), allogrooming is an indicator of consolation behavior. This result could be interpreted as PS exposure impaired consolation, which is an important part of social capabilities. To our knowledge, this is the first study to date to report the relationship between consolation and chronic PS stress in animals. The reduced awareness of others’ emotions, emotional withdrawal, or reluctance to respond to a distressed partner are frequently seen in psychiatric patients (Donges et al., 2005; Field et al., 2009; Young et al., 2015). Nevertheless, it is difficult to determine whether the reduced consolation in the defeated voles was due to the impaired emotion perception ability or just indifferent to their partners’ suffering. Further studies should be conducted to address this interesting question.
Studies in animals and humans suggest that the ACC plays important roles in empathy-like behavior (Morrison et al., 2004; Burkett et al., 2016). Consistent with these studies, we found substantial expression of c-Fos (a cellular marker of neural activity) within the ACC when the control voles (of both sexes) actively interacted with their stressed partners. At the same time, the reduced consolation behavior in the PS-exposed voles was accompanied by decreased c-Fos expression within the ACC.
Iniguez et al. provided convincing evidence that ES-exposed mice also exhibited depressive-like behavior, which resembles the behavioral profile of PS-exposed individuals (Warren et al., 2013; Iñiguez et al., 2018). However, we found that both behavioral performance and physiological responses (body weight variations, brain activation patterns, and receptor expression profiles in the ACC) were comparable between ES and control groups. Although we cannot exclude species differences (mice vs mandarin voles), the discrepancy may be largely due to methodological differences. In Iniguez’s studies (Warren et al., 2013; Iñiguez et al., 2018), the ES-exposed mice were housed next to a novel CD1 aggressor for an extended period of psychological stress after vicariously experiencing the defeat bouts. However, in our study, we returned the ES-exposed voles to their original cages and housed them with their partners to maintain their bonded relationship. Under this paradigm, social buffering effects may be induced (Smith and Wang, 2014; Donovan et al., 2018), therefore dampening the detrimental effect of ES. Nevertheless, at least some effects of ES exposure were visible. For example, the plasma CORT level of the ES group was higher than that of the control (Figure 6). Therefore, one must be cautious in directly comparing results from different studies when the study paradigms, animals, or other experimental conditions are not consistent.
OTR and V1aR
Recent studies have indicated that OT systems are involved in empathy-like behavior in both humans (Hurlemann et al., 2010) and rodents (Burkett et al., 2016; Li et al., 2019). In this study, we found that both OTR mRNA and protein expression were downregulated within the ACC in PS-exposed groups. The reduction in OTR may have been caused by repeated release of OT during the early period of chronic social defeat. Actually, stressors have been demonstrated to facilitate OT release (Smith and Wang, 2014), and increased OT release appears to desensitize and downregulate the OTR (Evans et al., 1997). The other evidence that OTR within the ACC was involved in the reduced consolation behavior of the PS-exposed voles is that microinjection of OT (10 ng) into the ACC rescued the impaired allogrooming behavior (Figure 9C), but such treatment had no significant effect on the nonstressed control voles, which may be due to the ceiling effect. Meanwhile, pretreatment with an OT receptor antagonist (decreased receptor activity) into the ACC attenuated the normal consolation behaviors of control voles (Figure 9O). Overall, the present study provides strong evidence that CSDS reduced consolation behaviors, possibly by reducing the abundance of OTRs in the ACC.
Another interesting finding was that 10 and 100 ng OT could also relieve social anxiety in SI (Figure 9D). The action of OT in brain regions such as the amygdala (Bale et al., 2001), paraventricular nucleus (PVN) (Smith and Wang, 2014), PrL (Sabihi et al., 2017), and accumbens nucleus (ACb) (Donovan et al., 2018) has been found to have an anxiolytic effect. The current study now adds the ACC to this list. However, the anxiolytic effect of OT was not observed in the OFT at any of the 3 selected dosages. Actually, a discrepancy between the SI and the OFT was also found in the following quinpirole, OTA, and raclopride treatments (Figure 9J–K, P–Q) and in other studies (Bale et al., 2001; Sabihi et al., 2014). This may be because the OFT is a model of anxiety-like behavior that features exploration and utilizes the anxiogenic stimulus of an open space, while the SI features anxiety in the social environment (Sabihi et al., 2014).
The brain AVP system is known to be involved in stress responses as well as in many social behaviors (Neumann and Landgraf, 2012). Cross-reactivity at the receptor level between OT and AVP has been described (Hicks et al., 2012). However, neither chronic PS nor ES exposure had a large effect on V1aR expression (mRNA or protein) within the ACC (Figures 7B and 8B). This result is in accordance with Donovan’s study (Donovan et al., 2018), which used an acute immobilization stress paradigm in prairie voles. Taken together, the data seem to suggest that the OT system plays a more important role than the AVP system in emotional (Sabihi et al., 2014) and social functions (Li et al., 2019) under both normal and stressed conditions within the ACC.
5-HT1AR
The current study indicated that 5-HT is involved in the reduced consolation behavior of the PS voles, possibly through postsynaptic 5-HT1ARs within the ACC. This is evident from (1) the decrease in 5-HT1AR density in the ACC with chronic PS exposure; (2) 5-HT1AR agonist 8-OH-DPAT (0.3 and 3 μg) administration rescued the reduced allogrooming behavior induced by PS exposure (Figure 9F); and (3) administration of the serotonin 1A receptor antagonist WAY-100635 attenuated consolation levels in control voles (Figure 9O). In a previous study, Kim et al. found that microinjection of 5-HT within the ACC reduced the freezing response of observing mice, which indicated impaired empathy (Kim et al., 2014). This seems contradictory to our results. However, it should be noted that there are many types of 5-HT receptors within the ACC other than 5-HT1AR. 5-HT administration may therefore activate other receptors within this brain region, confounding the results. This could be partially explained by the same finding that intra-ACC administration of a broad-spectrum 5-HT receptor antagonist, methysergide, had no effect on vicarious fear (Kim et al., 2014).
Although 2-week PS exposure significantly decreased 5-HT1AR density within the ACC (Figure 8C), the 5-HT1AR mRNA levels were not altered by the same treatment (Figure 7C). Such a discrepancy was also observed in D2R protein and mRNA expression (Figures 7E and 8E). It is well known that mRNAs are short-lived and that translation and posttranslational modifications occur before functional proteins are formed. A study indicated that the correlation coefficient between mRNA and protein measurements was less than 0.40 (Vogel and Marcotte, 2012). Therefore, the inconsistency between mRNA and protein changes in our study is not surprising.
The postsynaptic 5-HT1A receptors within the mPFC have been reported to exert antidepressant and anxiolytic effects (Artigas, 2013). In this study, we found that administration of 8-OH-DPAT (3 μg) in the ACC alleviated anxiety symptoms of the PS-exposed voles in both the SI and the OFT (Figures 9G–H), whereas injections of 5-HT1AR antagonist WAY-100635 increased anxiety-like behaviors in control voles (Figures 9P–Q). Similarly, Sartim et al. found that both PrL and IL 8-OH-DPAT administration significantly reduced the immobility time of rats in the forced swimming test, and this effect could be blocked by WAY100635 (Sartim et al., 2016). Furthermore, Fukumoto et al. found that stimulation of postsynaptic 5-HT1ARs is involved in both the acute and sustained antidepressant effects of ketamine (a rapid-acting nontraditional antidepressant) (Fukumoto et al., 2014, 2018). Nonetheless, further studies should be conducted to investigate whether the antidepressant and anxiolytic effects of 5-HT1AR are the same in the 3 subregions of the mPFC, which show different patterns of connectivity with subcortical and cortical structures and thus differentially contribute to a variety of emotional and behavioral processes (Hoover and Vertes, 2007; Sabihi et al., 2017).
D1Rs and D2Rs
In this study, we found that chronic PS exposure reduced DA D2R density within the ACC (Figure 8E) and that local administration of quinpirole (a selective DA D2R agonist, 10 ng) reversed the impaired consolation behavior (Figure 9I) and also social anxiety (Figure 9J) induced by chronic PS exposure. The same treatment had no such effect on control voles, which may exclude the nonspecific additive effects of the selected dosage (Figure 9L–M). Similarly, decreased receptor activity in control voles by injection of D2R receptor antagonist raclopride (0.5 μg) reduced the consolation levels (Figure 9O) and induced social anxiety (Figure 9P). These results are consistent with previous studies showing that ACC administration of haloperidol (another D2 receptor antagonist) but not SCH-23390 (a D1 receptor antagonist) decreased observational fear in mice (Kim et al., 2014) and an acute injection of quinpirole reversed social avoidance in mice subjected to chronic social defeat (Barik et al., 2013).
Although both D1Rs and D2Rs are sensitive to stress, we observed no changes in D1R expression between chronically PS-exposed animals and controls. The lack of change in D1R expression in the mPFC is consistent with previous studies showing that exposure to CSDS produced no change in mPFC D1R levels (Burke et al., 2011; Novick et al., 2011; Bagalkot et al., 2015). Although temporal changes in D1R abundance were observed with stress challenges (Avgustinovich and Alekseyenko, 2010), evidence suggests that D1Rs are preferentially recycled back to the plasma membrane (Bartlett et al., 2005), which may restore D1R numbers to the normal level. On the other hand, Huang et al. reported significantly decreased expression of D1Rs in the mPFC of CSDS-susceptible mice (Huang et al., 2016). The situation is much more complicated for D2Rs, the abundance of which has been variously reported to increase (Chen et al., 2013; Bagalkot et al., 2015), remain unchanged (Burke et al., 2011; Huang et al., 2016), and decrease (Wright et al., 2008; Suzuki et al., 2010) upon exposed to a variety of stressors. The discrepancies could be explained by different types/intensities/durations of stressors, species/sex/age differences, different subregions within the mPFC, methodological differences, or many other causes.
General Discussion and Conclusion
Overall, the results of this study demonstrated that chronic PS , but not ES exposure impaired consolation and induced depression-like behaviors in mandarin voles and OTR, 5-HT1AR, and D2R in the ACC may play important roles. It is worth mentioning that sexually dimorphic effects were barely found during these processes. This may provide additional evidence supporting the notion that comparable outcomes may allow males and females to similarly adapt to environmental challenges, particularly in species that adapt monogamous life strategies and require cooperative breeding. The limitations of this study are worth noting to avoid overstating the results. First, we did not determine the phase of the estrous cycle across the females utilized in our experiments. However, the effect of estrous cycle, if any, may affect all of the studied groups. Second, although the cannula placements were verified and injection rates were well controlled in the pharmacological studies, we cannot assure the drug did not diffuse to the adjacent brain regions and have its action on receptors there. Further studies targeting PrL or IL may actually help us answer this question. In spite of this, given the increased rates of mood-related illnesses across the globe, the strength of this study is that we have provided the basis for further studies into the mechanisms of impaired empathy in many psychiatric diseases, such as depression, autism, and schizophrenia.
Supplementary Material
Acknowledgments
We thank Yu-Ying Yang and Xin Zhang for assistance in conducting experiments and caring for voles and AJE for help in polishing the language in this manuscript.
This research was supported by the National Natural Science Foundation of China (31970424, 31670421, 31372213 and 31772473), the Natural Science Basic Research Plan in Shaanxin Province of China (2018JM3032) and the Fundamental Research Funds for Central University (GK201903059).
Interest Statement: None.
Author Contributions
Prof. Tai F.D. and R. Jia designed the study; L.F. Li conducted the majorities of experiments and wrote the original draft; W. Yuan participated in the ELISA experiment; Z.X. He and Z.M. Lian discussed the results and provided constructive comments; L.M. Wang participated in the immuno-fluorescence experiment; L.R. Meng, S.J. Zhu, H. Ma, Y.F. Xun, J. Zhang, W.Q. Cai, X.N. Zhang, and Q.Q. Guo participated in the behavior study and helped to collect and analyze the data. All authors contributed to and have approved the final manuscript.
References
- Artigas F. (2013) Serotonin receptors involved in antidepressant effects. Pharmacol Ther 137:119–131. [DOI] [PubMed] [Google Scholar]
- Avgustinovich DF, Alekseyenko OV (2010) [3H]SCH 23390 binding in various brain regions of C57BL/6J mice with repeated experience of victory or social defeat in agonistic interactions. Physiol Res 59:455–458. [DOI] [PubMed] [Google Scholar]
- Bagalkot TR, Jin HM, Prabhu VV, Muna SS, Cui Y, Yadav BK, Chae HJ, Chung YC (2015) Chronic social defeat stress increases dopamine D2 receptor dimerization in the prefrontal cortex of adult mice. Neuroscience 311:444–452. [DOI] [PubMed] [Google Scholar]
- Bale TL, Davis AM, Auger AP, Dorsa DM, McCarthy MM (2001) CNS region-specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. J Neurosci 21:2546–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barik J, Marti F, Morel C, Fernandez SP, Lanteri C, Godeheu G, Tassin JP, Mombereau C, Faure P, Tronche F (2013) Chronic stress triggers social aversion via glucocorticoid receptor in dopaminoceptive neurons. Science 339:332–335. [DOI] [PubMed] [Google Scholar]
- Bartlett SE, Enquist J, Hopf FW, Lee JH, Gladher F, Kharazia V, Waldhoer M, Mailliard WS, Armstrong R, Bonci A, Whistler JL (2005) Dopamine responsiveness is regulated by targeted sorting of D2 receptors. Proc Natl Acad Sci U S A 102:11521–11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M, Gray JA, Roth BL (2009) The expanded biology of serotonin. Annu Rev Med 60:355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke AR, Watt MJ, Forster GL (2011) Adolescent social defeat increases adult amphetamine conditioned place preference and alters D2 dopamine receptor expression. Neuroscience 197:269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett JP, Andari E, Johnson ZV, Curry DC, de Waal FB, Young LJ(2016) Oxytocin-dependent consolation behavior in rodents. Science (New York, NY) 351:375–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Yang JM, Hu TT, Xu TJ, Xu WP, Wei W (2013) Elevated dopamine D2 receptor in prefrontal cortex of CUMS rats is associated with downregulated cAMP-independent signaling pathway. Can J Physiol Pharmacol 91:750–758. [DOI] [PubMed] [Google Scholar]
- Cooper MA, McIntyre KE, Huhman KL (2008) Activation of 5-HT1A autoreceptors in the dorsal raphe nucleus reduces the behavioral consequences of social defeat. Psychoneuroendocrinology 33:1236–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean J, Keshavan M (2017) The neurobiology of depression: an integrated view. Asian J Psychiatr 27:101–111. [DOI] [PubMed] [Google Scholar]
- de Waal FBM, Preston SD (2017) Mammalian empathy: behavioural manifestations and neural basis. Nat Rev Neurosci 18:498–509. [DOI] [PubMed] [Google Scholar]
- Dong N, Du P, Hao X, He Z, Hou W, Wang L, Yuan W, Yang J, Jia R, Tai F (2017) Involvement of GABAA receptors in the regulation of social preference and emotional behaviors by oxytocin in the central amygdala of female mandarin voles. Neuropeptides 66:8–17. [DOI] [PubMed] [Google Scholar]
- Donges US, Kersting A, Dannlowski U, Lalee-Mentzel J, Arolt V, Suslow T (2005) Reduced awareness of others’ emotions in unipolar depressed patients. J Nerv Ment Dis 193:331–337. [DOI] [PubMed] [Google Scholar]
- Donovan M, Liu Y, Wang Z (2018) Anxiety-like behavior and neuropeptide receptor expression in male and female prairie voles: the effects of stress and social buffering. Behav Brain Res 342:70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JJ, Forrest-Owen W, McArdle CA (1997) Oxytocin receptor-mediated activation of phosphoinositidase C and elevation of cytosolic calcium in the gonadotrope-derived alphaT3-1 cell line. Endocrinology 138:2049–2055. [DOI] [PubMed] [Google Scholar]
- Field T, Diego M, Hernandez-Reif M (2009) Depressed mothers’ infants are less responsive to faces and voices. Infant Behav Dev 32:239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto K, Iijima M, Chaki S (2014) Serotonin-1A receptor stimulation mediates effects of a metabotropic glutamate 2/3 receptor antagonist, 2S-2-amino-2-(1S,2S-2-carboxycycloprop-1-yl)-3-(xanth-9-yl)propanoic acid (LY341495), and an N-methyl-D-aspartate receptor antagonist, ketamine, in the novelty-suppressed feeding test. Psychopharmacology (Berl) 231:2291–2298. [DOI] [PubMed] [Google Scholar]
- Fukumoto K, Iijima M, Funakoshi T, Chaki S (2018) Role of 5-HT1A receptor stimulation in the medial prefrontal cortex in the sustained antidepressant effects of ketamine. Int J Neuropsychopharmacol 21:371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammels C, Pishva E, De Vry J, van den Hove DL, Prickaerts J, van Winkel R, Selten JP, Lesch KP, Daskalakis NP, Steinbusch HW, van Os J, Kenis G, Rutten BP (2015) Defeat stress in rodents: from behavior to molecules. Neurosci Biobehav Rev 59:111–140. [DOI] [PubMed] [Google Scholar]
- Hicks C, Jorgensen W, Brown C, Fardell J, Koehbach J, Gruber CW, Kassiou M, Hunt GE, McGregor IS (2012) The nonpeptide oxytocin receptor agonist WAY 267,464: receptor-binding profile, prosocial effects and distribution of c-Fos expression in adolescent rats. J Neuroendocrinol 24:1012–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP (2007) Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct 212:149–179. [DOI] [PubMed] [Google Scholar]
- Huang GB, Zhao T, Gao XL, Zhang HX, Xu YM, Li H, Lv LX (2016) Effect of chronic social defeat stress on behaviors and dopamine receptor in adult mice. Prog Neuropsychopharmacol Biol Psychiatry 66:73–79. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Patin A, Onur OA, Cohen MX, Baumgartner T, Metzler S, Dziobek I, Gallinat J, Wagner M, Maier W, Kendrick KM (2010) Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J Neurosci 30:4999–5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Flores-Ramirez FJ, Riggs LM, Alipio JB, Garcia-Carachure I, Hernandez MA, Sanchez DO, Lobo MK, Serrano PA, Braren SH, Castillo SA (2018) Vicarious social defeat stress induces depression-related outcomes in female mice. Biol Psychiatry 83:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M, Baram TZ (2009) The neuro-symphony of stress. Nat Rev Neurosci 10:459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp JF. (2017) Depression is contagious: dyadic interventions may reduce infection. Am J Geriatr Psychiatry 25:396. [DOI] [PubMed] [Google Scholar]
- Kim BS, Lee J, Bang M, Seo BA, Khalid A, Jung MW, Jeon D (2014) Differential regulation of observational fear and neural oscillations by serotonin and dopamine in the mouse anterior cingulate cortex. Psychopharmacology (Berl) 231:4371–4381. [DOI] [PubMed] [Google Scholar]
- Li LF, Yuan W, He ZX, Wang LM, Jing XY, Zhang J, Yang Y, Guo QQ, Zhang XN, Cai WQ, Hou WJ, Jia R, Tai FD (2019) Involvement of oxytocin and GABA in consolation behavior elicited by socially defeated individuals in mandarin voles. Psychoneuroendocrinology 103:14–24. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang ZX (2003) Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience 121:537–544. [DOI] [PubMed] [Google Scholar]
- Meyza KZ, Bartal IB, Monfils MH, Panksepp JB, Knapska E (2017) The roots of empathy: through the lens of rodent models. Neurosci Biobehav Rev 76:216–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison I, Lloyd D, di Pellegrino G, Roberts N (2004) Vicarious responses to pain in anterior cingulate cortex: is empathy a multisensory issue? Cogn Affect Behav Neurosci 4:270–278. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Landgraf R (2012) Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci 35:649–659. [DOI] [PubMed] [Google Scholar]
- Novick AM, Forster GL, Tejani-Butt SM, Watt MJ (2011) Adolescent social defeat alters markers of adult dopaminergic function. Brain Res Bull 86:123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Manrique A, Gomila A (2018) The comparative study of empathy: sympathetic concern and empathic perspective-taking in non-human animals. Biol Rev Camb Philos Soc 93:248–269. [DOI] [PubMed] [Google Scholar]
- Pytka K, Podkowa K, Rapacz A, Podkowa A, Żmudzka E, Olczyk A, Sapa J, Filipek B (2016) The role of serotonergic, adrenergic and dopaminergic receptors in antidepressant-like effect. Pharmacol Rep 68:263–274. [DOI] [PubMed] [Google Scholar]
- Ramos L, Hicks C, Kevin R, Caminer A, Narlawar R, Kassiou M, McGregor IS (2013) Acute prosocial effects of oxytocin and vasopressin when given alone or in combination with 3,4-methylenedioxymethamphetamine in rats: involvement of the V1A receptor. Neuropsychopharmacology 38:2249–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabihi S, Durosko NE, Dong SM, Leuner B (2014) Oxytocin in the prelimbic medial prefrontal cortex reduces anxiety-like behavior in female and male rats. Psychoneuroendocrinology 45:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabihi S, Dong SM, Maurer SD, Post C, Leuner B (2017) Oxytocin in the medial prefrontal cortex attenuates anxiety: anatomical and receptor specificity and mechanism of action. Neuropharmacology 125:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartim AG, Guimarães FS, Joca SR (2016) Antidepressant-like effect of cannabidiol injection into the ventral medial prefrontal cortex-Possible involvement of 5-HT1A and CB1 receptors. Behav Brain Res 303:218–227. [DOI] [PubMed] [Google Scholar]
- Smith AS, Wang Z (2014) Hypothalamic oxytocin mediates social buffering of the stress response. Biol Psychiatry 76:281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Han SD, Lucas LR (2010) Chronic passive exposure to aggression decreases D2 and 5-HT 1B receptor densities. Physiol Behav 99:562–570. [DOI] [PubMed] [Google Scholar]
- Tai FD, Wang TZ(2001) Social organization of mandarin voles in burrow system. Acta Theriol Sin 21:50–56. [Google Scholar]
- Tse YC, Lopez J, Moquin A, Wong SA, Maysinger D, Wong TP (2019) The susceptibility to chronic social defeat stress is related to low hippocampal extrasynaptic NMDA receptor function. Neuropsychopharmacology 44:1310–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel C, Marcotte EM (2012) Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet 13:227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhu Z, Hou W, Zhang X, He Z, Yuan W, Yang Y, Zhang S, Jia R, Tai F (2019) Serotonin signaling trough prelimbic 5-HT1A receptors modulates CSDS-induced behavioral changes in adult female voles. Int J Neuropsychopharmacol 22:208–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren BL, Vialou VF, Iñiguez SD, Alcantara LF, Wright KN, Feng J, Kennedy PJ, Laplant Q, Shen L, Nestler EJ, Bolaños-Guzmán CA (2013) Neurobiological sequelae of witnessing stressful events in adult mice. Biol Psychiatry 73:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt MJ, Roberts CL, Scholl JL, Meyer DL, Miiller LC, Barr JL, Novick AM, Renner KJ, Forster GL (2014) Decreased prefrontal cortex dopamine activity following adolescent social defeat in male rats: role of dopamine D2 receptors. Psychopharmacology (Berl) 231:1627–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright LD, Hébert KE, Perrot-Sinal TS (2008) Periadolescent stress exposure exerts long-term effects on adult stress responding and expression of prefrontal dopamine receptors in male and female rats. Psychoneuroendocrinology 33:130–142. [DOI] [PubMed] [Google Scholar]
- Young KS, Parsons CE, Stein A, Kringelbach ML (2015) Motion and emotion: depression reduces psychomotor performance and alters affective movements in caregiving interactions. Front Behav Neurosci 9:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, An S, Tai F, Zhang X, He F, Wang J, An X, Wu R (2012) The effects of neonatal paternal deprivation on pair bonding, NAcc dopamine receptor mRNA expression and serum corticosterone in mandarin voles. Horm Behav 61:669–677. [DOI] [PubMed] [Google Scholar]
- Zoratto F, Sbriccoli M, Martinelli A, Glennon JC, Macrì S, Laviola G (2018) Intranasal oxytocin administration promotes emotional contagion and reduces aggression in a mouse model of callousness. Neuropharmacology 143:250–267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.