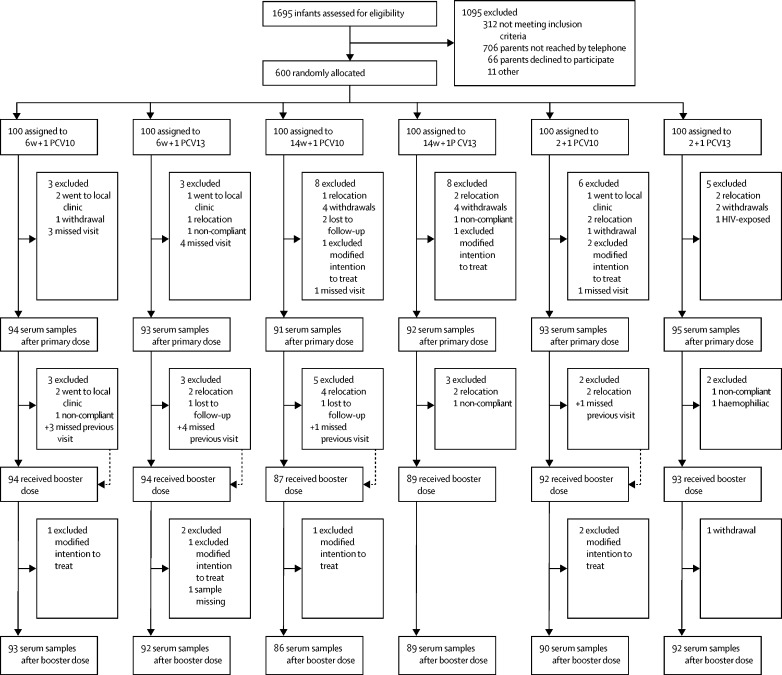
Figure 1.
Trial profile
Participants reincluded are indicated with a + symbol. Infants were randomly assigned to receive one primary dose of PCV10 or PCV13 at age 6 weeks (6w + 1 PCV10 and PCV13 groups) or 14 weeks (14w + 1 PCV10 and PCV13 groups) or two primary doses, one each at ages 6 weeks and 14 weeks (2 + 1 groups). All infants received a booster dose at age 40 weeks. PCV10=ten-valent pneumococcal conjugate vaccine. PCV13=13-valent pneumococcal conjugate vaccine.