Abstract
Background
Leukemia represents about 5% of all human cancers. Despite advances in therapeutics, a substantial number of patients succumb to the disease. Several subtypes of leukemia are inherently more resistant to treatment despite intensive chemotherapy or targeted therapy.
Methods
Here we describe the generation of T cell engaging (CD3) bispecific antibodies (BsAbs) built on humanized IgG frameworks using the IgG(L)-scFv format against two targets expressed on acute lymphoblastic leukemia (ALL) and on acute myeloid leukemia (AML).
Results
Each BsAb mediated potent anti-leukemia effect against ALL (CD19) and AML (CD33) in vitro and in xenograft models. Importantly, the CD19-specific BsAb (BC250) was effective against hematogenous spread preventing metastases to liver and kidney in mice bearing ALL and Burkitt’s lymphoma xenografts. BC250 was more potent than the The Food and Drug Administration (FDA)-approved BsAb blinatumomab against ALL xenografts in vivo as measured by tumor bioluminescence and mouse survival. Furthermore, the combination of the CD19 and CD33 BsAbs in two xenograft models of mixed phenotype acute leukemia (biphenotypic and bilineal leukemia) was far superior than monotherapy with either of the BsAbs alone.
Conclusions
Selective combinations of these leukemia-specific BsAb offer the potential to overcome tumor heterogeneity or clonal escape in the modern era of antibody-based T cell-driven immunotherapy.
Keywords: antibodies, hematologic neoplasms, immunotherapy, T-lymphocytes, antigens, neoplasm
Introduction
Leukemia is the most common cancer in children and its treatment in adults remains suboptimal. Although the advent of chimeric antigen receptor (CAR), bispecific antibodies (BsAbs) and targeted therapies has dramatically improved the outlook among these patients; survival remains poor among patients who relapse.1
Similar to solid cancers, both acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL) are composed of heterogenous populations of cells that evolve during treatment where the composition of tumor cells could significantly differ before and after treatment or relapse, typically with dire therapeutic implications.2–5 In addition, mixed phenotype leukemia is composed of heterogenous populations of cells with different lineage phenotype including surface marker expression.6–8 With the advent of immunotherapy, leukemia clones could downregulate antigen expression, take advantage of splice variants, and even structural mutations.9–11 Antigen heterogeneity aside, leukemia could also exploit regulatory T cells that circulate in the blood of patients with AML, shown to be associated with poorer prognosis and resistance to therapy.12 13 Myeloid-derived suppressor cells constitute another family of cells in tumor microenvironment that can blunt immune surveillance; they have been shown to be expanded in patients with AML and to dampen the anti-leukemia immune response.14 15
Among the many resistance mechanisms, antigenic coverage seems the most logical next step. Myeloid leukemia switch is now recognized as an escape from CD19-chimeric antigen receptor T cell (CART) therapy.16 We propose to test the combination of T cell-based therapies directed at two lineage-specific antigens, using a tetravalent IgG(L)-scFv platform (anti-tumor IgG with anti-CD3 scFv fused to the light chains).17 In this manuscript, we generated BsAb built on humanized IgG frameworks directed at leukemia-associated antigens, CD19 and CD33. While monotherapy with each BsAb was potent in cytotoxicity assays and in xenograft models of leukemia, only the combination therapy was effective against mixed lineage leukemias.
Methods
Generation of BsAbs
The murine FMC63 anti-CD19 antibody18 was humanized by grafting the heavy chain complementarity determining region (CDR) sequences onto the human framework IGHV4-4*08-IGHJ4*01, and the light chain CDR sequences onto the human framework IGKV1-33*01-IGKJ2*01. The CDRs of the heavy and light chains of murine anti-CD33 My96 antibody19 were grafted onto human IgG1 frameworks based on their homology with human frameworks IGHV1-46*01- IGHJ6*01 for VH, IGKV4-1*01-IGKJ4*01 for VL, respectively. All VH and VL domains have a combined average humanness of more than 85% (table 1).
Table 1.
The humanness of anti-CD19 and CD33 antibodies
| VH humanness | VL humanness | |
| Anti-CD19 agents humanness | ||
| CD19 CAR (FMC63) | 63.6 | 73.7 |
| Glenmark hFMC63 antibody | 81.8 | 88.4 |
| BC250 | 84.5 | 86.3 |
| Anti-CD33 agents humanness | ||
| Mylotarg | 71.4 | 78 |
| BC269 | 85.7 | 93 |
CAR, chimeric antigen receptor.
The anti-CD19 BsAb (BC250) and anti-CD33 BsAb (BC269) were designed using VH/VL domains from the corresponding humanized antibodies and huOKT3 scFv20 fused to the C-terminus of the light chain of a human IgG1 based on a method described previously.21 22 To remove glycosylation and complement binding, the N297A and K322A mutations in the Fc region were made, respectively. Several anti-CD19 BsAb humanized variants with different affinities for CD19 were generated during a single humanization campaign and named as BC253, BC254, and BC255. BsAbs were expressed by transient transfection of Expi293F cells (ThermoFisher Scientific - Waltham, MA, USA) and purified using protein-A affinity columns using ÄKTA fast protein liquid chromatography (GE Healthcare - Chicago, IL, USA). Purity was assessed by high performance liquid chromatography. Blinatumomab was obtained from the Memorial Sloan Kettering Cancer Center (MSKCC) pharmacy.
Cytotoxicity assay (chromium51 release assay)
Leukemia cells were cultured in RPMI1640 (Corning Scientific - Corning, NY, USA) supplemented with 10% fetal bovine serum (Life Technologies - Carlsbad, CA, USA) at 37°C in a 5% CO2 humidified incubator. Cytotoxicity assay was performed as described before.23
Cell lines and fluorescence activated cell sorting (FACS) analysis
NALM6-luciferase cells were provided by Renier J. Brentjens at MSKCC. CD19-negative NALM6-luciferase cells were provided by David Scheinberg (MSKCC). These cells were transduced with human CD33 gene whose construct was provided by Trinidad Hernández-Caselles (IMIB-University of Murcia).24
BV173-luciferase cells were provided by R.J. O’Reilly (MSKCC). Raji and Daudi cells were transduced with retroviral virus to express click beetle luciferase (kindly provided by Vladimir Ponomarev (MSKCC). JIH-5 cells were bought from DSMZ (Leibniz, Germany). MOLM13-luciferase cells were provided by David Scheinberg (MSKCC).
Anti-human antibodies against CD3, CD4, CD8, CD19, and CD33 (Biolegend - San Diego, CA, USA) were used to stain cells. Stained cells were processed with a FACScalibur instrument (BD Biosciences - San Jose, CA, USA) and analyzed with FlowJo software (FlowJo, Ashland, Oregon, USA).
In vivo studies
All mouse experiments were performed in compliance with the Institutional Animal Care and Use Committee guidelines. The immunodeficient NOD.Cg-Prkdcscid IL-2R-γtm1Wjl/SzJ (NSG) mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA) and provided with Sulfatrim food. For tumor challenge experiments, 6–10-week-old mice were inoculated with leukemic cells intravenously with (0.5–1×106 cells). For intravenous-leukemia model, activated human T cells (ATC) that were in culture for 7–9 days were admixed with the BsAb and were intravenously injected weekly for 1–3 weeks starting 3–6 days after tumor inoculation. BC119 (CD3xGD2 BsAb) was used as a control since GD2 is not expressed by any of the leukemia cell lines used. One thousand international unit of interleukin 2 (IL-2) was subcutaneously injected two times per week to support T cell persistence. Tumor growth was monitored using the IVIS bioluminescence imager.
Pharmacokinetics assessment
Five 12-week-old female C57BL/6 mice were injected retroorbitally with 100 µg BC250. Blood was collected at 4, 8, 24, 48, 72, and 96 hours after BC250 administration. Immediately after collection blood was centrifuged at 800g for 10 min to separate serum and serum was frozen at −80°C. Serum antibody concentration was measured using ELISA. Briefly, huCD3 was adsorbed onto 96-well microtiter plates to capture the serum BC250 which was detected with peroxidase-conjugated mouse anti-human IgG1 (Jackson ImmunoResearch - West Grove, PA, USA) using o-phenylenediamine dihydrochloride as substrate.
Statistical analysis
Statistical analyses were performed using Graphpad Prism software. Comparison between two groups were performed using a Student’s t-test and comparisons between multiple groups were determined by analysis of variance (ANOVA) with Tukey correction and multiple comparison analysis. A p value of <0.05 was considered statistically significant. Error bars denote the SEM. Survival analysis was performed using Log-rank test.
Results
CD19-BsAb-mediated antibody-dependent T cell-mediated cytotoxicity (ADTC) against ALL cell line at femtomolar EC50 in vitro
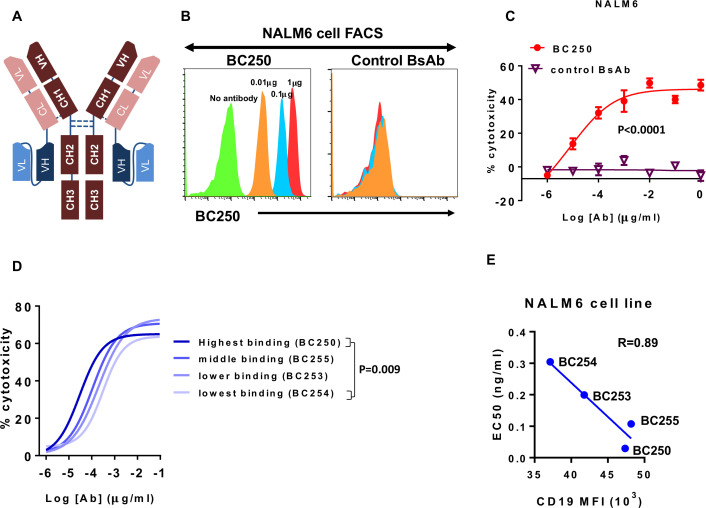
BsAb engaging T cells toward CD19 on ALL were made using the IgG(L)-scFv platform (figure 1A). The humanness of published humanized CD19 antibodies is summarized in table 1. These BsAb bound to CD19(+) ALL cells, carrying out ADTC at femtomolar EC50 concentrations (figure 1B, C). Antibody binding avidity, measured by flow cytometry correlated with ADTC potency (EC50) in cytotoxicity assays (figure 1D, E). Clone BC250 demonstrated the highest binding and potency against CD19(+) leukemia and was chosen for further testing (figure 1D).
Figure 1.
T cell engaging bispecific antibody (BsAb) against CD19 lyses acute lymphoblastic leukemia cell line with femtomolar EC50. (A) Schematic view of the IgG-scFv BsAb. (B) NALM6 CD19(+) cells were stained with the T cell-engaging CD19-specific BsAb BC250 or a control BsAb. (C) ATC were cultured with 51Cr-labeled NALM6 cells (E:T ratio=10) in the presence of different doses of BC250 or a control BsAb for 4 hours. Release of 51Cr was used as an indicator of cytotoxicity. EC50s were compared using t-test. (D–E) T cell-engaging BsAbs with different affinities for CD19 were tested in a T cell-mediated cytotoxicity assay. NALM6 cells were stained with the same antibodies. The EC50 (from cytotoxicity assays, with comparison between EC50s using ANOVA and multiple comparison analysis) and CD19 mean fluorescence intensity or mean fluorescence intensity (MFI) (from flow cytometry experiments) were plotted against each other. ATC, activated human T cells; ANOVA, analysis of variance.
BsAb targeting CD19 and polyclonal T cells could ablate ALL xenografts in vivo
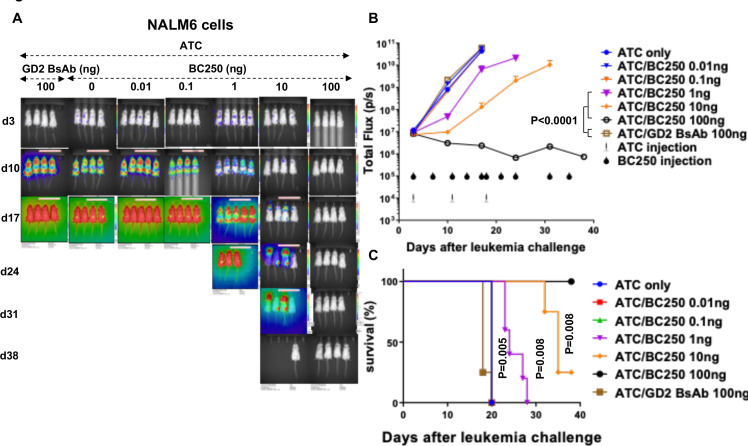
To test the in vivo potency of BC250, NSG mice were inoculated intravenously with CD19(+) NALM6 ALL cells. After 3 days, treatment was started with weekly intravenous injections of 10 million human ATC co-administered with decreasing doses of BC250 two times per week. One hundred nanogram of BC250 in the presence of ATC was curative while 100 ng of control BsAb specific for GD2 (which is not expressed on NALM6) or ATC alone had no effect. Survival of the BC250-treated mice was also significantly improved (figure 2).
Figure 2.
A low dose of the CD19-specific BsAb redirects polyclonal T cells to treat ALL xenografts in vivo. (A–C) Immunodeficient NSG mice were intravenously inoculated with CD19(+) NALM6-luciferase human ALL xenografts (0.5×106 cells). Therapy was started after 3 days. Mice received weekly injections of 107 ATC with different doses of BC250. In the control groups, mice either received ATCs or a control GD2 BsAb with ATCs. Leukemia growth was monitored using the IVIS bioluminescent imager (A–B) and survival was assessed over time. Area under the curve of the log total flux until day 17 was compared between groups using ANOVA and multiple comparison analysis (C). All mice received subcutaneous injections of interleukin-2 (1000 IU/two times/week). The experiment with NALM6 cells was repeated two times. ALL, acute lymphoblastic leukemia; ATC, activated human Tcells; BsAb, bispecific antibody; ANOVA, analysis of variance.
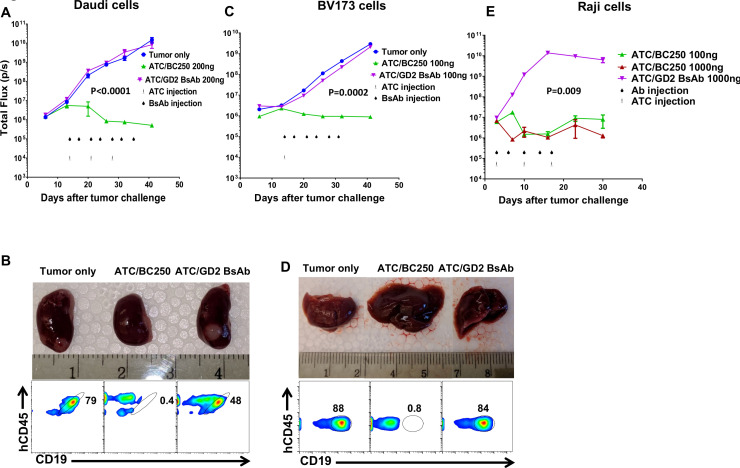
To evaluate the potency of BC250 across various CD19(+) lymphoid malignancies, NSG mice were inoculated intravenously with B-cell lymphoma cell lines Daudi or Raji, or the B cell precursor leukemia cell line BV173. Treatment was started after 3 days (Raji) or 14 days (Daudi and BV173). While the combination of human ATC with BC250 treated leukemic mice, control GD2 BsAb was completely ineffective similar to the no treatment group (figure 3 and online supplemental figure 1). The dose of T cells required for leukemia control varied with tumor model depending on the doubling time of xenografts. For BV173 cells, a single dose of 9 million T cells followed by six doses of BC250 was enough to cure leukemia, while three doses of T cells and BC250 were needed to control Daudi and Raji cells in mice. Furthermore, flow cytometry assessment of kidney and liver showed CD19(+) leukemia/lymphoma (Daudi and BV173) in untreated and in control BsAb/ATC-treated mice; in contrast, no tumor cells were detected in the BC250/ATC groups (figure 3B, D).
Figure 3.
The combination of BC250 and T cells treats mice in several ALL xenograft mouse models in vivo. (A–E) Immunodeficient NSG mice were intravenously inoculated with CD19(+) Daudi-luciferase (106), BV173-luciferase (106), or Raji-luciferase (0.5×106). Treatment was started after 3 ((E), Raji) or 14 ((A) and (C), Daudi and BV173) days of leukemia engraftment. Treatment consisted of ATC injections (three doses each 20×106 for Daudi, one dose of 8.8×106 for BV173, or three doses each 10×106 for Raji) with BC250 (CD3xCD19 BsAb) or a GD2 BsAb control antibody. Interleukin two was administered two times per week (1000 IU) to support ATC persistence. Leukemia growth was monitored using the IVIS bioluminescent imager. 39 days after leukemia injection, mice were sacrificed and kidney (Daudi group) or liver (BV173) were analyzed by flow cytometry for the presence of leukemic cells (B and D). The experiment with Raji cells was repeated two times. Area under the curve of the log total flux was compared between groups using ANOVA and multiple comparison analysis. ALL, acute lymphoblastic leukemia; ATC, activated human T cells; BsAb, bispecific antibody; ANOVA, analysis of variance.
jitc-2020-001626supp001.pdf (316.5KB, pdf)
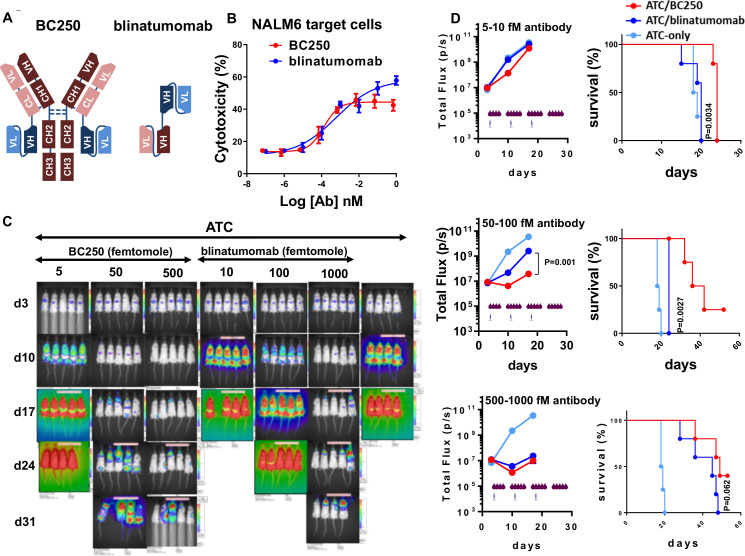
BC250 was superior to blinatumomab against ALL xenografts in vivo
Blinatumomab (Blincyto) is a tandem scFv bispecific T cell engager (BiTE) against CD19 and the only Food and Drug Administration (FDA)-approved BsAb against ALL and lymphoma (figure 4A). The potency of BC250 and blinatumomab were compared in ADTC (figure 4B). In vitro, BC250 and blinatumomab appear to have very similar potencies (EC50, 0.65 vs 3 pM), although blinatumomab demonstrated slightly higher maximum killing at the higher antibody concentrations (figure 4B). To compare the potency of the drugs in vivo, NSG mice inoculated with NALM6 human ALL cells were treated with BsAbs in combination with ATC. To compensate for the monovalent CD19/CD3 binding of blinatumomab versus bivalent binding of BC250 to CD19/CD3, a twofold higher molar dose of blinatumomab was administered. In addition, to compensate for the short in vivo half-life of blinatumomab, both antibodies were injected daily. As shown in figure 4C, D, as low as 5 fmol of BC250 could reduce leukemia growth and improve mice survival while 10 fmol of blinatumomab did not have any benefit over the T cell-only control group (figure 4D top panels, p<0.0001 and p=0.006 for tumor signal at days 10 and 17; p=0.0034 for survival). For the second dose group (50–100 fmol), there was a significant benefit of BC250 over blinatumomab for both leukemia burden and survival (figure 4D middle panel, p=0.001 for tumor signal at days 10 and 17; p=0.0027 for survival). Higher doses of both BsAbs could significantly reduce the leukemia growth, however, BC250 seemed superior in reducing the leukemia growth and survival extension (figure 4D lower panel, p=0.01 for tumor signal at day 10; p=0.062 for survival).
Figure 4.
BC250 outperforms blinatumomab for treatment of ALL xenografts in vivo. (A) Schematic of the structure of BC250 and blinatumomab. (B) ATC were cultured with 51Cr-labeled NALM6 cells (E:T ratio=10) in the presence of different doses of BC250 or blinatumomab for 4 hours. release of 51Cr was used as an indicator of cytotoxicity. (C–D) Immunodeficient NSG mice were intravenously inoculated with CD19(+) NALM6-luciferase human ALL xenografts (0.5×106 cells). Therapy was started after 4 days. Mice received weekly injections of 107 ATC with different doses of BC250 or blinatumomab. Antibody injection was performed 5 days per week intraperitoneally. In the control groups, mice received only ATCs without any antibody. Leukemia growth was monitored using the IVIS bioluminescent imager. All mice received subcutaneous injection of interleukin-2 (1000 IU/two times/week). The comparison between BC250 and Blinatumumab was repeated two times. Area under the curve of the log total flux was compared between groups using ANOVA and multiple comparison analysis. Survival analysis was done by Log-rank test. ALL, acute lymphoblastic leukemia; ATC, activated human T cells; BsAb, bispecific antibody; ANOVA, analysis of variance.
Superior potency of BC250 over blinatumomab is more than its favorable PK
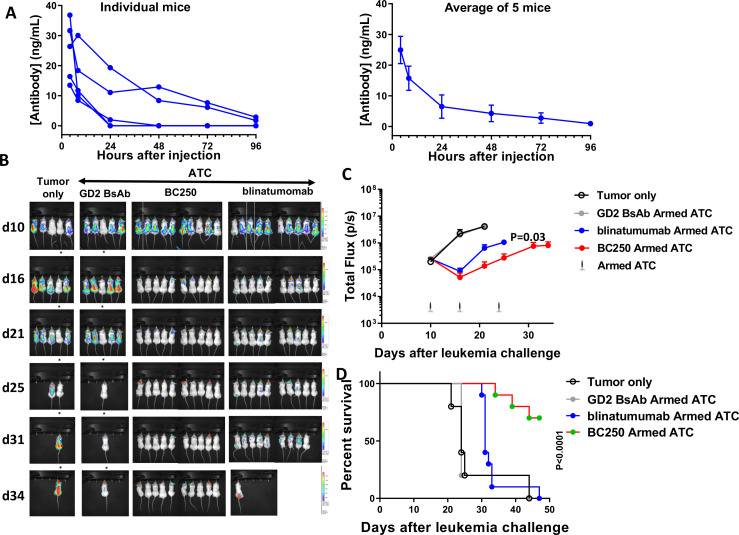
Blinatumomab is a 54 KD protein whose molecular weight is below the kidney clearance (70 kD) and therefore it has a poor PK with an elimination half-life (T1/2) of 2.1 hours (blinatumomab FDA label). To assess the mechanism of BC250’s superior in vivo potency, we assessed the PK of BC250 in mice (figure 5A). The T1/2 of BC250 in this experiment was ~19 hours, approximately nine times the T1/2 of blinatumomab. BC250 remained detectable in two of five mice for up to 96 hours post injection. To investigate other potential mechanisms of BC250’s superior potency, we conducted an in vivo armed-T cell study. Activated T cells were mixed with either BC250 or blinatumomab for 20 min in a small volume of media (50 µL) and the unbound antibody was washed away. Equimolar doses of BC250 and blinatumomab were chosen, corrected for the bivalency of BC250 versus the univalent structure of blinatumomab, meaning that two times as many moles of blinatumomab were used compared with BC250 to arm the T cells. Armed-T cells were injected into mice that had been inoculated with NALM6 leukemia 10 days prior. BC250 armed-T cells reduced tumor burden 4.7-fold and 3.8-fold by days 21 (p=0.03) and 25 (p=0.017), respectively, and extended the survival of the leukemic mice (7/10 BC250 mice alive on day 47 versus 0/10 blinatumomab mice, p<0.0001) (figure 5D). These data demonstrate that the superiority of BC250 over blinatumomab cannot be entirely attributed to its more favorable PK and suggest that bivalent binding of BC250 versus monovalent binding of blinatumomab plays an important role in vivo.
Figure 5.
BC250 versus blinatumomab has superior pharmacokinetics and in vivo potency in animal models (A) Serum concentration of BC250 in vivo. Left panel—individual mice, right panel—average of group, error bars=SEM. (B–C) NSG mice were intravenously inoculated with CD19(+) NALM6-luciferase human ALL xenografts (0.5×106 cells). Mice were treated with 107 ATC armed with either an irrelevant control antibody (GD2 antibody), BC250, or blinatumomab once weekly for 3 weeks, and subcutaneous IL-2 (1000 IU) two times per week for 3 weeks. A control group was inoculated with tumor but did not receive any treatment. Leukemia growth was monitored using the IVIS bioluminescent imager. Area under the curve of the log total flux until day 21 was compared between groups using ANOVA and multiple comparison analysis (B–C) and survival was assessed over time and analyzed using Log-rank test (D). ALL, acute lymphoblastic leukemia; ATC, activated human T cells; BsAb, bispecific antibody; IL, interleukin; ANOVA, analysis of variance.
Combining BsAb specific for CD19 and CD33 for biphenotypic leukemia
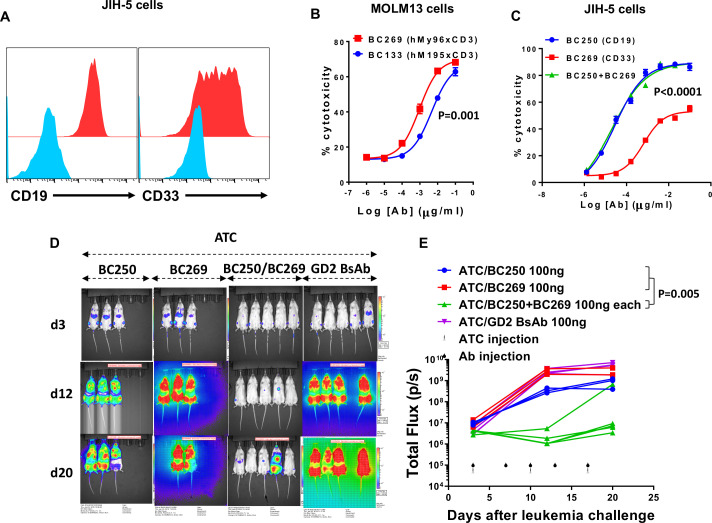
Mixed-phenotype acute leukemia (MPAL) is a heterogeneous type of acute leukemia with the expression of myeloid and lymphoid markers on a single type of blast cells (biphenotypic acute leukemia, BAL) or a discrete admixed population of myeloid and lymphoid blasts (bilineal leukemia).25 B/myeloid leukemia is the most common type of MPAL.2 JIH-5 is a human B lymphoid BAL cell line with homogenous expression of CD19 on all cells and variable expression of CD33 on subpopulations (figure 6A). We previously reported a humanized T cell-engaging BsAb against CD33 (BC133) based on the CD3 clone M195 with potent anti-AML function in vitro and in vivo.17 23 However, the CD3xCD33 BsAb we reported in this manuscript (BC269) has 24-fold higher affinity to CD33 and improved cytotoxicity in ADTC assays (fivefold lower EC50, figure 6B) and a better humanness score (humanness score of 93% vs 76.6% for VL and 85.7% vs 76.5% for VH for BC269 vs BC133, respectively) (table 2). To test the effect of combined BsAb therapy, the susceptibility of this cell line to monotherapy or combination therapy with anti-CD19 (BC250) and anti-CD33 (BC269) BsAbs was assessed in ADTC. The maximum killing of BC269 was about 50% of the maximum killing of BC269/BC250 combination therapy, consistent with the partial expression of CD33 on BAL cells (figure 6C). Since CD19 was expressed on all JIH-5 cells, BC250 monotherapy was as effective as BC250/BC269 combination therapy. To test the combination therapy of BsAbs against BAL, NSG mice were inoculated with a mixture of CD19(−)CD33(+) and CD19(+)CD33(−) NALM6 cells. Whereas treatment with either BC250 or BC269 in the presence of ATC did not prevent tumor growth, combination therapy of the same two BsAbs showed strong anti-leukemia responses (figure 6D, E).
Figure 6.
Combination of T cell-engaging anti-CD19 and anti-CD33 BsAbs can treat biphenotypic leukemia. (A) JIH-5 cells were stained with anti-CD19 and anti-CD33 antibodies. (B) ATC were cultured with 51Cr-labeled MOLM13 in the presence of different doses of BC2133 or BC269. Release of 51Cr was used as an indicator of cytotoxicity. EC50s were compared using a Student’s t-test. (C) ATC were cultured with 51Cr-labeled JIH-5 in the presence of different doses of BC250, BC269, or a combination of two antibodies. Release of 51Cr was used as an indicator of cytotoxicity. EC50s were compared using ANOVA. (D–E) Immunodeficient NSG mice were intravenously inoculated with 0.5×106 NALM6 CD19(+)CD33(−) and 0.5×106 NALM6 CD19(−)CD33(+) human ALL xenografts. Therapy was started after 3 days. Mice received weekly injections of 107 ATC with either monotherapy or combination therapy of BC250 and BC269. GD2 BsAb was used as a control antibody. Leukemia growth was monitored using the IVIS bioluminescent imager. Area under the curve of the log total flux was compared between groups using ANOVA and multiple comparison analysis. ALL, acute lymphoblastic leukemia; ATC, activated human T cells; BsAb, bispecific antibody; ANOVA, analysis of variance.
Table 2.
Affinity comparison between BC269 and BC133 BsAbs at 37°C by surface plasmon resonance
| VH humanness% | VL humanness% | ka (1/Ms) | kd (1/s) | KD (M) | EC50 (μg/ml) | |
| BC269 | 85.7 | 93 | 7.84E+05 | 6.58E−04 | 1.02E−10 | 0.0009467 |
| BC133 | 76.5 | 76.6 | 9.85E+05 | 7.97E−03 | 2.47E−09 | 0.004632 |
BsAb, bispecific antibody.
Combining CD19-BsAb and CD33-BsAb in T cell therapy of bilineal leukemia
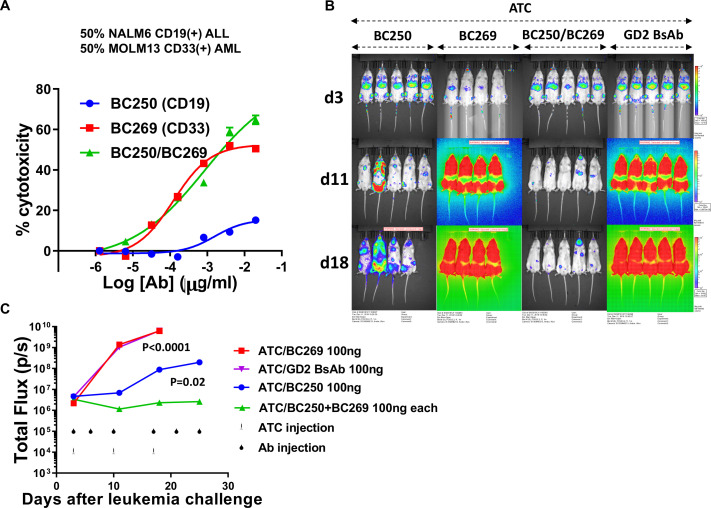
Another type of MPAL is acute bilineal leukemia where discrete admixed populations of myeloid and lymphoid blasts coexist in the same patient. In ADTC assays where target cells were composed of a mixture of CD19(+) NALM6 and CD33(+) MOLM13 cells, the combination of BC250 and BC269 showed an additive effect at higher antibody concentrations (figure 7A). In a xenograft model of bilineal leukemia generated by intravenous injection of equal numbers of CD19(+) ALL and CD33(+) AML cells, the combination of CD19-BsAb and CD33-BsAb suppressed tumor growth, in contrast to groups treated with either BC250 or BC269 alone (figure 7B, C).
Figure 7.
Combination of T cell-engaging anti-CD19 and anti-CD33 BsAbs can treat bilineal leukemia. (A) ATC were cultured with a 50%–50% mixture of 51Cr-labeled CD19(+) NALM6 and CD33(+) MOLM13 cells in the presence of different doses of BC250, BC269, or a combination of both. Release of 51Cr was used as an indicator of cytotoxicity. (B–C) Immunodeficient NSG mice were intravenously inoculated with 0.5×106 NALM6 CD19(+) ALL and 0.5×106 MOLM13 CD33(+) AML xenografts. Therapy was started after 3 days. Mice received weekly injections of 107 ATC with either monotherapy or combination therapy of BC250 and BC269. GD2 BsAb was used as a control antibody. Leukemia growth was monitored using the IVIS bioluminescent imager. For statistical analysis, one mouse with the highest BLI signal was censored from analysis. Area under the curve of the log total flux until day 18 was compared between groups using ANOVA and multiple comparison analysis. ALL, acute lymphoblastic leukemia; ATC, activated human T cells; BsAb, bispecific antibody; ANOVA, analysis of variance.
Discussion
Most adults with ALL relapse after achieving a complete remission and the majority of patients succumb to leukemia, however, about 25% never achieve a complete remission at all.26 For AML, the prognosis is even worse. In this manuscript, we reported the generation of two humanized T cell engaging BsAbs against CD19 and CD33 using a tetravalent IgG(L)-scFv platform (anti-tumor IgG with anti-CD3 scFv fused to the light chains),17 one of the most potent BsAb platforms. Placing tumor and T-cell binding domains on the same side of a BsAb (cis configuration) elicits substantially stronger anti-tumor activity, in vitro and in vivo, compared with positioning them on opposite sides (trans configuration). Moreover, using two cis-modules in the same BsAb further improved cytotoxicity (up to 2000-fold). Additionally, separating antigen-binding components with a single Ig domain (CL) dramatically enhanced cytokine release and in vivo tumor responses compared with smaller (G4S1) or larger (CH1–CH2–CH3) spacers.
In this manuscript we show that whereas each antibody alone was able to retarget polyclonal T cells to treat antigen(+) target cells, the combination of these antibodies was successful in treating various forms of MPAL (bilineal and biphenotypic leukemia) in animal models. In the case of CD19-BsAb (BC250), a comparison was made against blinatumomab, the only FDA-approved BsAb to treat leukemia/lymphoma. BC250 was more potent than blinatumomab in reducing tumor burden and in increasing survival of leukemic mice using a daily injection regimen to compensate for the fast pharmacokinetics of blinatumomab (2 hour serum half-life).27 Using T cells armed ex vivo with BsAb (thereby excluding the confounding effect of poor PK of blinatumomab), we proved the definitive advantage of BC250 over blinatumomab in vivo. Since in these T cell-arming experiments any unbound BsAb was removed, T cell surface-bound BC250 was able to outperform surface-bound blinatumomab, a property we have previously described,17 and has since been validated in a number of BsAbs targeting CD33, GD2, Her2, and GPA33 using this unique IgG-L-scFv format.21 23 28 29 In addition to its superior potency, unlike blinatumomab, our anti-CD19 VH had 84.5% humanness while VL had 86.3% humanness, vs 69.4% and 65.3% for the humanness of blinatumomab’s VH and VL, respectively), which translates into a reduction in immunogenicity and neutralizing antibody generation, potential hurdles for retreatment with blinatumomab or current mouse scFv-based CD19 CART. To assess the effect of antibody affinity on its potency, we tested four different humanized versions of the CD19 BsAb in T cell dependent cytotoxicity assays. Importantly, there was a correlation between antibody affinity and potency, where BC250 with the highest affinity (lowest kD) was the most potent (lowest EC50). The effect of affinity on potency has previously been reported for the BiTE format.30 However, there was also a ceiling beyond which further affinity maturation did not improve ADTC in vitro or in vivo.29 Our results confirmed the affinity range (100 nM to 0.1 nM) where improvements can be expected.31–33
The FDA approval of CD19 CART cells paved the way to improve survival rates of patients with ALL. Despite efforts to increase the accessibility of CART therapies by generation of off-the-shelf allogeneic CART cells,34 the current FDA-approved CD19 CART products are primarily derived from autologous sources, where the associated manufacturing complexity prevents this therapeutic modality to be used as an off-the-shelf treatment. The processing time required for cell manufacturing can make CART cells unsuitable for at least 30% of patients.35 Furthermore, unintentional transduction of leukemic cells with the CAR gene during manufacturing has been associated with CD19 recognition blockade leading to leukemia relapse.36 In addition, the long-term persistence of CART cells may prevent normal hematopoiesis following the induction of remission and in the case of CD19 leading to prolonged B-cell cytopenia and hypogammaglobulinemia.37 38 Furthermore, CARTs against myeloid leukemia have yet to be FDA approved. Mono-specific CARTs could not prevent relapse related to lineage switch, epitope loss or splice variants, and they cannot be used to treat patients with MPAL.
Other mechanisms of leukemia escape after CART or BsAb treatment have recently emerged. One such mechanism, lineage switch, has been reported in patients with B-ALL developing mixed lineage leukemia harboring the MLL gene after CD19 CART or T cell-engaging BsAb blinatumomab therapies.6–8 In addition, leukemia relapse secondary to the selection for pre-existing CD19 spliced variants lacking the CD19 epitope or CD19 loss could be found in up to 20% of patients treated with CD19 CART cells or blinatumomab.10 39 40 Furthermore, those leukemias carrying different markers on distinct lineages are not expected to be curable with a single antibody alone. The use of dual-specific CART cells that express two different scFvs specific for two separate leukemia antigens might be an alternative; however, it may not easily permit balanced or controlled expression of one scFv over the other. Here, the fine tuning of the two specificities as the leukemia lineages contract or expand could be difficult to orchestrate. Mixing two or more CART cell populations could be another alternative; however, this will add more complexity to an already demanding manufacturing process. With dual-specific CARs toxicity could mount and inclusion of separate suicide genes for each CART product can further complicate their clinical development. In addition, these suicide genes are not available in the current FDA-approved CART cell products or been adequately tested in large enough sample size. On the other hand, combination antibody therapy is now acceptable in immunooncology, although combination BsAb strategies will need more careful consideration.41 It is conceivable that the dose of each antibody can be adjusted for each indication, while each can be individually interrupted or stopped, to reduce side effects or on disappearance of the cell population targeted by the antibody (eg, in case of MPAL). BsAbs with potency for two different tumor associated antigens have been generated42; however, selection of clones with dual specificity for both antigens and the potential of cross reactivity to other antigens complicates generation of such antibodies. Besides, the dose of antibody for each target could not be adjusted separately. The combination of BsAbs to target various antigens on tumor cells could reduce the chance of relapse. For example, combining CD19 and CD22 BsAbs could reduce the chance of CD19(−) relapse of patients with B-cell malignancies.
One of the important complications of CD19 CART and blinatumomab therapy is the development of neurotoxicity. We did not observe any neurological adverse effects in mice treated with BC250 or blinatumomab. One of the suggested mechanisms of neurotoxicity is the expression of CD19 on brain pericytes43; however, the lack of cross-reactivity of these antibodies to mouse CD19 did not allow us to assess this phenomenon in our experiments. Nevertheless, we do not expect BC250 format to cross the blood brain barrier due to its larger size as we have not observed toxicity with other BsAb built on the same format even when the target is on mouse brain (unpublished results from GD2xCD3 BsAb). Hence, a limitation of our BsAb is that it may not treat central nervous system (CNS) leukemia, unlike blinatumomab or CD19 CART cells.
To conclude, we demonstrated that by combining powerful anti-leukemia BsAbs based on the IgG(L)-scFv platform for common lymphoid and myeloid markers (CD19 and CD33) the T cell-based treatment of ALL, AML, and MPAL could be improved.
Acknowledgments
We thank Hong Xu for doing the Biacore experiments and Mahiuddin Ahmed for help with designing humanized sequences. We also thank Brian Santich, Yi Feng, and Hoa Tran for their technical support.
Footnotes
SSH and ME-C contributed equally.
Contributors: SSH and ME-C conceived the study, performed the experiments, analyzed the data, designed the figures, and wrote the manuscript; H-fG helped with antibody generation; N-KVC conceived the study, supervised the project, analyzed the data, and wrote and edited the manuscript.
Funding: This work was supported in part by Funds from Enid A. Haupt Endowed Chair, Kids Walk for Kids with Cancer NYC, Isabella Santos Foundation, Katie Find a Cure Foundation, the Robert Steel Foundation, NIH/NCI Cancer Center Support Grant P30 CA008748, and sponsored research agreement from Ymabs Therapeutics Inc.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Yu J, Wang W, Huang H. Efficacy and safety of bispecific T-cell engager (BiTE) antibody blinatumomab for the treatment of relapsed/refractory acute lymphoblastic leukemia and non-Hodgkin's lymphoma: a systemic review and meta-analysis. Hematology 2019;24:199–207. 10.1080/16078454.2018.1549802 [DOI] [PubMed] [Google Scholar]
- 2.Yan L, Ping N, Zhu M, et al. . Clinical, immunophenotypic, cytogenetic, and molecular genetic features in 117 adult patients with mixed-phenotype acute leukemia defined by WHO-2008 classification. Haematologica 2012;97:1708–12. 10.3324/haematol.2012.064485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding L, Ley TJ, Larson DE, et al. . Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature 2012;481:506–10. 10.1038/nature10738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walter MJ, Shen D, Ding L, et al. . Clonal architecture of secondary acute myeloid leukemia. N Engl J Med 2012;366:1090–8. 10.1056/NEJMoa1106968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quesada AE, Hu Z, Routbort MJ, et al. . Mixed phenotype acute leukemia contains heterogeneous genetic mutations by next-generation sequencing. Oncotarget 2018;9:8441–9. 10.18632/oncotarget.23878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gardner R, Wu D, Cherian S, et al. . Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood 2016;127:2406–10. 10.1182/blood-2015-08-665547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacoby E, Nguyen SM, Fountaine TJ, et al. . CD19 CAR immune pressure induces B-precursor acute lymphoblastic leukaemia lineage switch exposing inherent leukaemic plasticity. Nat Commun 2016;7:12320. 10.1038/ncomms12320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rayes A, McMasters RL, O'Brien MM. Lineage switch in MLL-rearranged infant leukemia following CD19-Directed therapy. Pediatr Blood Cancer 2016;63:1113–5. 10.1002/pbc.25953 [DOI] [PubMed] [Google Scholar]
- 9.Topp MS, Gökbuget N, Zugmaier G, et al. . Phase II trial of the anti-CD19 bispecific T Cell–Engager Blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-Precursor acute lymphoblastic leukemia. JCO 2014;32:4134–40. 10.1200/JCO.2014.56.3247 [DOI] [PubMed] [Google Scholar]
- 10.Sotillo E, Barrett DM, Black KL, et al. . Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov 2015;5:1282–95. 10.1158/2159-8290.CD-15-1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orlando EJ, Han X, Tribouley C, et al. . Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat Med 2018;24:1504–6. 10.1038/s41591-018-0146-z [DOI] [PubMed] [Google Scholar]
- 12.Shenghui Z, Yixiang H, Jianbo W, et al. . Elevated frequencies of CD4⁺ CD25⁺ CD127lo regulatory T cells is associated to poor prognosis in patients with acute myeloid leukemia. Int J Cancer 2011;129:1373–81. 10.1002/ijc.25791 [DOI] [PubMed] [Google Scholar]
- 13.Szczepanski MJ, Szajnik M, Czystowska M, et al. . Increased frequency and suppression by regulatory T cells in patients with acute myelogenous leukemia. Clin Cancer Res 2009;15:3325–32. 10.1158/1078-0432.CCR-08-3010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pyzer AR, Avigan DE, Rosenblatt J, et al. . Clinical trials of dendritic cell-based cancer vaccines in hematologic malignancies. Hum Vaccin Immunother 2014;10:3125–31. 10.4161/21645515.2014.982993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehta RS, Chen X, Antony J, et al. . Myeloid Derived Suppressor Cells (MDSC)-like Acute Myeloid Leukemia (AML) Cells Are Associated with Resistance to Cytotoxic Effects of Autologous (Auto) T-Lymphocytes (CTLs). Biology of Blood and Marrow Transplantation 2015;21:S191–2. 10.1016/j.bbmt.2014.11.290 [DOI] [Google Scholar]
- 16.Perna F, Sadelain M. Myeloid leukemia switch as immune escape from CD19 chimeric antigen receptor (CAR) therapy. Transl Cancer Res 2016;5:S221–5. 10.21037/tcr.2016.08.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santich BH, Park JA, Tran H, et al. . Interdomain spacing and spatial configuration drive the potency of IgG-[L]-scFv T cell bispecific antibodies. Sci Transl Med 2020;12. 10.1126/scitranslmed.aax1315. [Epub ahead of print: 11 03 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zola H, MacArdle PJ, Bradford T, et al. . Preparation and characterization of a chimeric CD19 monoclonal antibody. Immunol Cell Biol 1991;69 (Pt 6):411–22. 10.1038/icb.1991.58 [DOI] [PubMed] [Google Scholar]
- 19.Kenderian SS, Ruella M, Shestova O, et al. . CD33-specific chimeric antigen receptor T cells exhibit potent preclinical activity against human acute myeloid leukemia. Leukemia 2015;29:1637–47. 10.1038/leu.2015.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woodle ES, Thistlethwaite JR, Jolliffe LK, et al. . Humanized OKT3 antibodies: successful transfer of immune modulating properties and idiotype expression. J Immunol 1992;148:2756–63. [PubMed] [Google Scholar]
- 21.Xu H, Cheng M, Guo H, et al. . Retargeting T cells to GD2 pentasaccharide on human tumors using bispecific humanized antibody. Cancer Immunol Res 2015;3:266–77. 10.1158/2326-6066.CIR-14-0230-T [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng M, Ahmed M, Xu H, et al. . Structural design of disialoganglioside GD2 and CD3-bispecific antibodies to redirect T cells for tumor therapy. Int J Cancer 2015;136:476–86. 10.1002/ijc.29007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoseini SS, Guo H, Wu Z, et al. . A potent tetravalent T-cell-engaging bispecific antibody against CD33 in acute myeloid leukemia. Blood Adv 2018;2:1250–8. 10.1182/bloodadvances.2017014373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pérez-Oliva AB, Martínez-Esparza M, Vicente-Fernández JJ, et al. . Epitope mapping, expression and post-translational modifications of two isoforms of CD33 (CD33M and CD33m) on lymphoid and myeloid human cells. Glycobiology 2011;21:757–70. 10.1093/glycob/cwq220 [DOI] [PubMed] [Google Scholar]
- 25.Charles NJ, Boyer DF. Mixed-Phenotype acute leukemia: diagnostic criteria and pitfalls. Arch Pathol Lab Med 2017;141:1462–8. 10.5858/arpa.2017-0218-RA [DOI] [PubMed] [Google Scholar]
- 26.Fielding AK, Richards SM, Chopra R, et al. . Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood 2007;109:944–50. 10.1182/blood-2006-05-018192 [DOI] [PubMed] [Google Scholar]
- 27.Wu B, Hijazi Y, Wolf A, et al. . Pharmacokinetics (pK) of blinatumomab and its clinical implications. JCO 2013;31:3048–9. 10.1200/jco.2013.31.15_suppl.3048 [DOI] [Google Scholar]
- 28.Lopez-Albaitero A, Xu H, Guo H, et al. . Overcoming resistance to HER2-targeted therapy with a novel HER2/CD3 bispecific antibody. Oncoimmunology 2017;6:e1267891. 10.1080/2162402X.2016.1267891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Z, Guo H-F, Xu H, et al. . Development of a tetravalent Anti-GPA33/Anti-CD3 bispecific antibody for colorectal cancers. Mol Cancer Ther 2018;17:2164–75. 10.1158/1535-7163.MCT-18-0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng M, et al. Successful engineering of a highly potent single-chain variable-fragment (scFv) bispecific antibody to target disialoganglioside (GD2) positive tumors. Oncoimmunology 5, e1168557 2016. [DOI] [PMC free article] [PubMed]
- 31.Liddy N, Bossi G, Adams KJ, et al. . Monoclonal TCR-redirected tumor cell killing. Nat Med 2012;18:980–7. 10.1038/nm.2764 [DOI] [PubMed] [Google Scholar]
- 32.Root A, Cao W, Li B, et al. . Development of PF-06671008, a highly potent anti-P-cadherin/anti-CD3 bispecific DART molecule with extended half-life for the treatment of cancer. Antibodies 2016;5:6 10.3390/antib5010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reusch U, Harrington KH, Gudgeon CJ, et al. . Characterization of CD33/CD3 tetravalent bispecific tandem diabodies (TandAbs) for the treatment of acute myeloid leukemia. Clin Cancer Res 2016;22:5829–38. 10.1158/1078-0432.CCR-16-0350 [DOI] [PubMed] [Google Scholar]
- 34.Brudno JN, Somerville RPT, Shi V, et al. . Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J Clin Oncol 2016;34:1112–21. 10.1200/JCO.2015.64.5929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuster SJ, Bishop MR, Tam CS, et al. . Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 2019;380:45–56. 10.1056/NEJMoa1804980 [DOI] [PubMed] [Google Scholar]
- 36.Ruella M, Xu J, Barrett DM, et al. . Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Nat Med 2018;24:1499–503. 10.1038/s41591-018-0201-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kochenderfer JN, Dudley ME, Feldman SA, et al. . B-Cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 2012;119:2709–20. 10.1182/blood-2011-10-384388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalos M, Levine BL, Porter DL, et al. . T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med 2011;3:ra73. 10.1126/scitranslmed.3002842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maude SL, Frey N, Shaw PA, et al. . Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371:1507–17. 10.1056/NEJMoa1407222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Topp MS, Gökbuget N, Zugmaier G, et al. . Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol 2014;32:4134–40. 10.1200/JCO.2014.56.3247 [DOI] [PubMed] [Google Scholar]
- 41.Henricks LM, Schellens JHM, Huitema ADR, et al. . The use of combinations of monoclonal antibodies in clinical oncology. Cancer Treat Rev 2015;41:859–67. 10.1016/j.ctrv.2015.10.008 [DOI] [PubMed] [Google Scholar]
- 42.Schaefer G, Haber L, Crocker LM, et al. . A two-in-one antibody against HER3 and EGFR has superior inhibitory activity compared with monospecific antibodies. Cancer Cell 2011;20:472–86. 10.1016/j.ccr.2011.09.003 [DOI] [PubMed] [Google Scholar]
- 43.Beck JD, Birtel M, Haefner E, et al. . CIMT 2019: report on the 17th annual meeting of the association for cancer immunotherapy. Hum Vaccin Immunother 2020;16:808–15. 10.1080/21645515.2019.1675459 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2020-001626supp001.pdf (316.5KB, pdf)