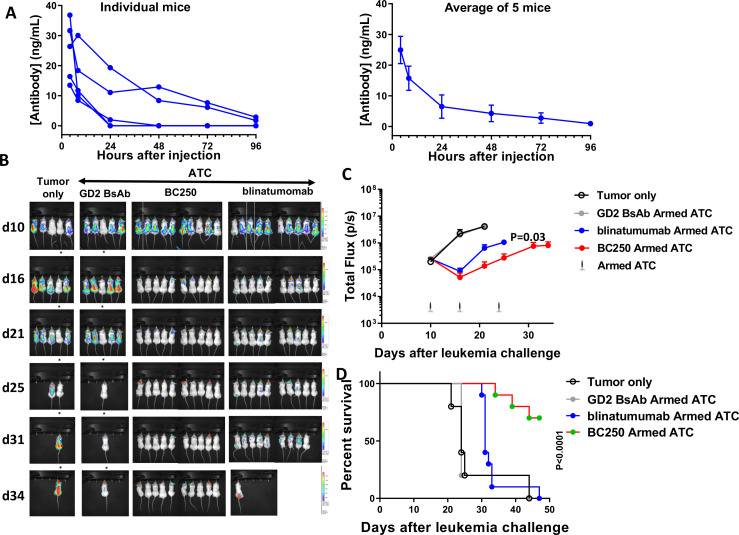
Figure 5.
BC250 versus blinatumomab has superior pharmacokinetics and in vivo potency in animal models (A) Serum concentration of BC250 in vivo. Left panel—individual mice, right panel—average of group, error bars=SEM. (B–C) NSG mice were intravenously inoculated with CD19(+) NALM6-luciferase human ALL xenografts (0.5×106 cells). Mice were treated with 107 ATC armed with either an irrelevant control antibody (GD2 antibody), BC250, or blinatumomab once weekly for 3 weeks, and subcutaneous IL-2 (1000 IU) two times per week for 3 weeks. A control group was inoculated with tumor but did not receive any treatment. Leukemia growth was monitored using the IVIS bioluminescent imager. Area under the curve of the log total flux until day 21 was compared between groups using ANOVA and multiple comparison analysis (B–C) and survival was assessed over time and analyzed using Log-rank test (D). ALL, acute lymphoblastic leukemia; ATC, activated human T cells; BsAb, bispecific antibody; IL, interleukin; ANOVA, analysis of variance.