Fig. 6.
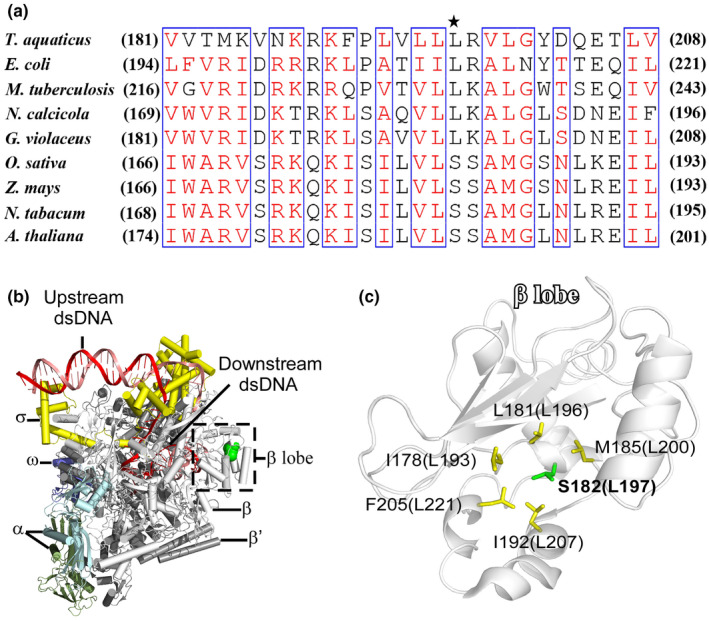
RNA editing at rpoB‐545 likely facilitates proper protein folding of the plastid‐encoded RNA polymerase (RNAP) β‐subunit. (a) Partial amino acid sequence alignment of RNAP β‐subunits of five bacterial species reveals a conserved leucine at the position corresponding to the edited codon (codon 182) of the rice chloroplast β‐subunit. By contrast, sequence alignment of chloroplast β‐subunits of four plant species reveals a serine at these positions (denoted by the asterisk). T. aquaticus, Thermus aquaticus; E. coli, Escherichia coli; M. tuberculosis, Mycobacterium tuberculosis; N. calcicola, Nostoc calcicola; G. violaceus, Gloeobacter violaceus; O. sativa, Oryza sativa; Z. mays, Zea mays; N. tabacum, Nicotiana tabacum; A. thaliana, Arabidopsis thaliana. (b) The crystal structure of T. aquaticus RNAP‐promoter open complex shows that L197 is located within the β‐lobe domain (dashed box) of the RNAP β‐subunit. The RNAP α, β, β′ and ω subunits are shown in cyan, gray, dark gray and blue, respectively, the promoter DNA is shown in red, and the σ‐factor is shown in yellow. (c) Amino acid L197 is buried inside the hydrophobic core of the β‐lobe domain. Numbers of amino acid residues correspond to the positions in the T. aquaticus RpoB protein. The equivalent positions in the O. sativa protein are given in parentheses.
