Fig. 1.
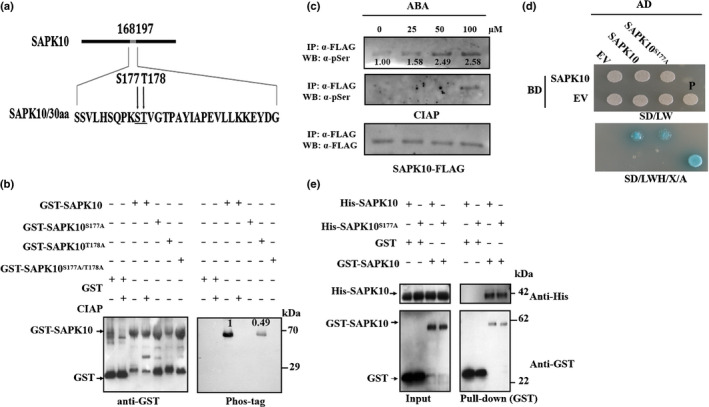
SAPK10 is autophosphorylated on serine 177. (a) Schematic representation of the putative autophosphorylation sites of SAPK10. Ser‐177 and Thr‐178 in the T‐ loop region of SAPK10 (amino acids 168–197) are indicated in bold. (b) Serine 177 is the key phosphosite of SAPK10. Equal amounts of GST‐SAPK10 and other mutated versions (GST‐SAPK10S177A, GST‐SAPK10T178A, GST‐SAPK10S177A/T178A) were detected with anti‐GST antibody (left panel) and the phosphorylated proteins were detected with biotinylated Phos‐tag™ zinc BTL111 complex (right panel). The GST protein was used as negative control. GST, glutathione S‐transferase. (c) SAPK10 phosphorylation was enhanced under abscisic acid (ABA) treatment. Two‐week‐old pro35S:SAPK10‐FLAG seedlings (Nipponbare, Oryza sativa ssp. japonica) were treated with ABA in different concentrations (0, 25, 50 and 100 μM) for 6 h. Equal amounts of immunoprecipitated SAPK10‐FLAG proteins were immunoblotted against antiphosphoserine (a‐pSer) antibody (top panel), treated with calf intestinal phosphatase (CIAP) at 37°C for 30 min and against a‐pSer antibody (middle panel) and anti‐FLAG antibody (bottom panel). The relative intensity of SAPK10‐FLAG under no‐ABA treatment was set to 1.00. (d) Yeast two‐hybrid assay. Yeast cells cotransformed with SAPK10 or SAPK10S177A fused to the GAL4 activation domain (SAPK10‐AD or SAPK10S177A‐AD) and SAPK10 fused to the GAL4‐binding domain (SAPK10‐BD) were grown on selective media. BD, pGBKT7; AD, pGADT7; EV, empty vector; SD/LW, ‐Leu‐Trp; SD/LWH, ‐Leu‐Trp‐His; P, positive control using pGADT7‐T + pGBKT7‐53; X: X‐α‐Gal in 0.04 mg ml−1; a, Aureobasidin A in 100 ng mI−1. (e) Pull‐down assay. Purified His‐SAPK10, His‐SAPK10S177A, GST and GST‐SAPK10 were subjected to pull‐down assays and detected with anti‐His and anti‐GST antibodies, respectively. Molecular mass markers are shown (kDa).
