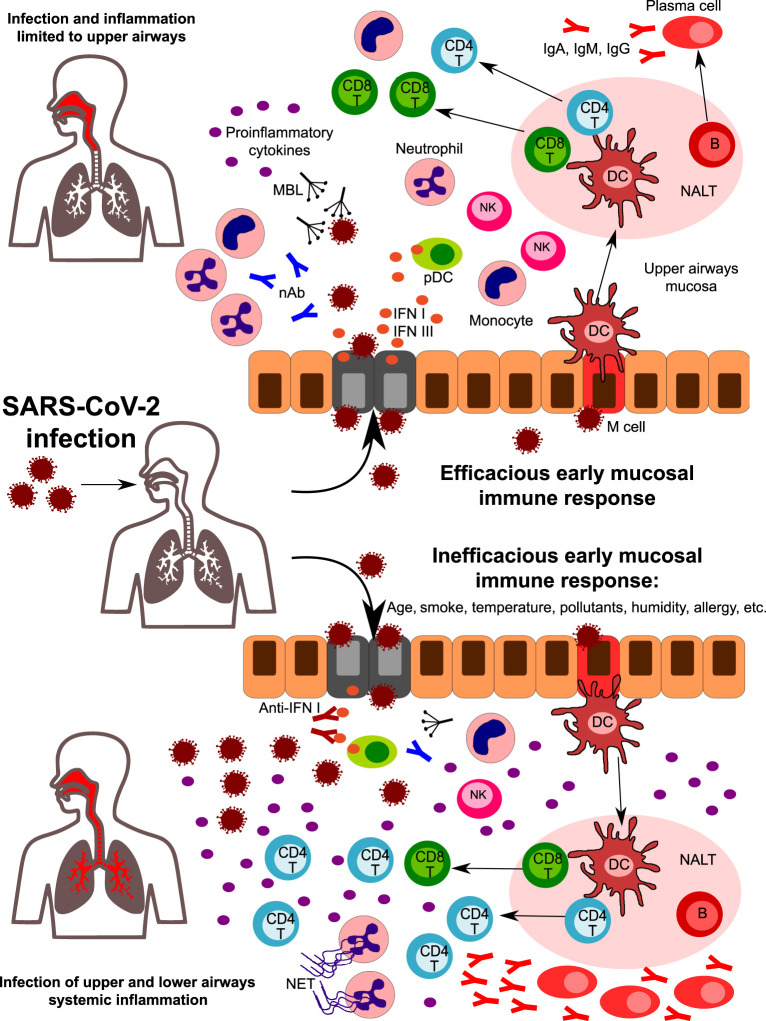
Fig. 3.
A hypothetical role for the early mucosal response in the upper airways in SARS-CoV-2 infection progression. Following SARS-CoV-2 entrance in the nasal or oral cavity, epithelial cells of the upper airways are the primary target site of infection. Innate immunity components in the upper airway mucosa are responsible for the first line of defense. Humoral components such as natural antibodies (nAb) and lectins (including mannose-binding lectin, MBL) can recognize glycoside structures of the virus, while epithelial infected cells and plasmacytoid DC release high amounts of type I IFNs, that are crucial in the initial antiviral response. Cells of innate immunity are activated by viral PAMPS and by the release of DAMPS from infected cells. An efficacious early mucosal innate response allows the development of adaptive immunity, with the expansion of CD4 + helper and CD8 + cytotoxic T cells and the differentiation of Ig-secreting plasma cells. Coordinated innate and adaptive responses contribute to the final elimination of the pathogen (upper part). However, factors such as age, smoke, pollutants, temperature, humidity, and genetics can affect the early response, leading to an inefficacious control of viral replication (lower part). Reduced levels of nAb and/or MBL, the presence of autoantibodies neutralizing type I IFN activity, and of impaired development of antigen-specific CD8 + T cells are some of the factors that can predispose to uncontrolled viral propagation and infection of lower airways. Viral escape is accompanied by a huge release of pro-inflammatory cytokines, with the recruitment and expansion of several subsets of innate and adaptive immunity. Neutrophils accumulate in the lungs and are massively activated, leading to NETs formation. All together, these mechanisms amplify the inflammatory response, leading to uncontrolled systemic inflammation.