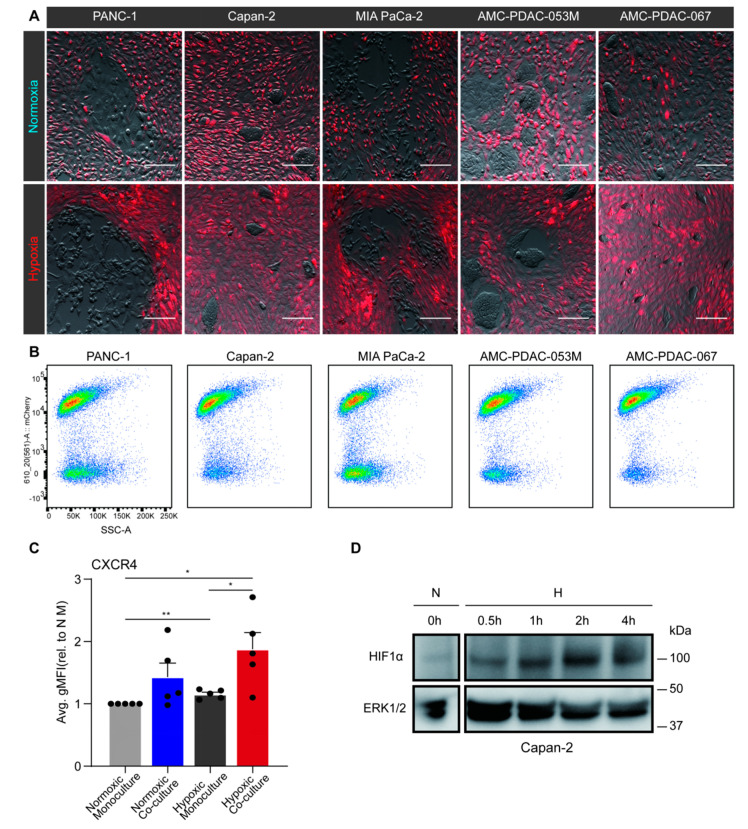
Figure 2.
Hypoxia-activated pancreatic stellate cells (PSCs) promote epithelial-to-mesenchymal transition (EMT) in pancreatic ductal adenocarcinoma (PDAC) cells. (A) PS1 stellate cells were transduced with an mCherry fluorescent construct (red). Next, untransduced PANC-1, Capan-2, MIA PaCa-2, AMC-PDAC-053M, AMC-PDAC-067 PDAC cells were either co-cultured with mCherry-labeled PS1 or monocultured for 96 h under normoxia or hypoxia. Images are shown of co-cultured cells after 96-h normoxia (upper row) or hypoxia (lower row; brightness and contrast of the images were adjusted). Scale bar: 50 µm. (B) Flow cytometry cytoplot examples of the co-cultures as shown in panel A. Populations in the cytoplots indicate mCherry-positive PS1 cells and negative PDAC cells (mCherry signal is on Y-axis). Cells were dissociated from the coculture prior to fluorescence-activated cell sorting, separating the PS1 cells and PDAC cells. (C) CXCR4 expression of PDAC cells was determined by flow cytometry. Values represent the average gMFI ± S.E.M. of all PDAC cell lines and were normalized to normoxic monoculture group (* p < 0.05, ** p < 0.01). Each dot refers to a cell line (PANC-1, Capan-2, MIA PaCa-2, AMC-PDAC-053M, AMC-PDAC-067). (D) Capan-2 cells were incubated under hypoxic conditions for indicated times with normoxic incubation as a control. Cells were processed for Western blotting, using antibodies against HIF1α. ERK1/2 was used as loading control.