Abstract
Introduction
Recently, the COVID-19 pandemic has spread globally, necessitating the development of new methods for its prevention and treatment. The purpose of this study was to evaluate the antiviral activity of photodynamic therapy (PDT) against SARS-CoV-2 in vitro.
Methods
Vero E6 cells and SARS-CoV-2 isolated in Russia were used for PDT with methylene blue (MB) and Radachlorin. A continuous laser with wavelength λ = 662 nm in doses of 16 J/cm2 and 40 J/cm2 laser irradiation was used for PDT of a viral suspension and SARS-CoV-2-infected cells. The direct cytopathogenic effect of SARS-CoV-2 was evaluated via light microscopy to calculate the TCID50 in the samples and perform statistical analysis.
Results
Viral suspensions of SARS-CoV-2 that had a TCID50 greater than 103 were inactivated by PDT in the presence of MB and Radachlorin. Vero E6 cells were protected from 104 TCID50 of SARS-CoV-2 by PDT post infection. The range of protective concentrations was 1.0–10.0 μg/ml and 0.5–5.0 μg/ml for MB and Radachlorin, respectively. Additionally, it was found that MB and Radachlorin also possess significant antiviral activity even without PDT. The 50 % inhibitory concentration (IC50) against 102 TCID50 of SARS-CoV-2 was found to be 0.22 and 0.33 μg/mL with the addition of MB and Radachlorin, respectively, to cells concomitantly with virus, whereas in the case of applying the photosensitizers at 3.5 h post infection, the IC50 was 0.6 and 2.0 μg/mL for MB and Radachlorin, respectively.
Conclusion
PDT shows high antiviral activity against SARS-CoV-2 when combined with MB and Radachlorin in vitro.
Keywords: COVID-19, SARS-CoV-2, Antiviral photodynamic therapy, Methylene blue, Radachlorin
1. Introduction
Over the past two decades, different coronavirus species have caused three severe human disease outbreaks characterized by respiratory tract lesions [1]. The COVID-19 pandemic, caused by a new coronavirus, has spread rapidly across the world. Modern sequencing techniques have made it possible to decode viral genomes and rapidly carry out their taxonomic identification [2]. The virus was classified as a novel coronavirus and named SARS-CoV-2, belonging to the family Coronaviridae of the order Nidovirales. As of 05 December, SARS-CoV-2 has circulated in more than 219 countries, with 66,310,189 registered cases of human infection and 1,526,234 deaths [3]. The development of the COVID-19 pandemic was characterized, especially initially, by the absence of antiviral drugs and vaccines to prevent and treat the infection. The high infection rate of SARS-CoV-2 is also associated with severe clinical problems, such as the development of viral bilateral pneumonia and pronounced immunopathological changes, causing high mortality, especially among elderly individuals and in patients suffering from comorbidities [4].
The severity of COVID-19 urgently requires the development of new methods for the prevention and treatment of the disease. One of these approaches can be antiviral photodynamic therapy [5]. Previously, the effectiveness of PDT in the inactivation of mammalian viruses has been demonstrated for several RNA- and DNA-containing viruses. Some of these viruses include herpesviruses; human immunodeficiency virus; hepatitis A, B, and C viruses; human parvovirus B19; human cytomegalovirus; adenoviruses; and enteroviruses. It has also been observed that enveloped viruses are more sensitive to PDT than nonenveloped viruses [[6], [7], [8]]. Currently, PDT is widely used to inactivate viruses in different biological fluids, for example, in blood tissue samples. PDT is also highly effective for the treatment of surface viral lesions on the skin and mucous membranes [9,10].
Many photosensitizers (PSs) made from various chemical compounds are used for PDT [5]; among these are methylene blue (MB) and Radachlorin, which are categorized as medicinal drugs in the Russian Federation. MB has long been used against various infectious agents, especially in urology [11,12]. MB is a phenothiazine compound that possesses photosensitizer properties. Radachlorin contains 70–90 % chlorin E6, derived from the chlorophyll of microalgae belonging to the genus Spirulina. Radachlorin is a second-generation PS with a maximum absorption of light energy in the red spectrum at a wavelength of 662 nm. It is widely used for treating precancerous virus-associated cervical intraepithelial neoplasms as well as cancerous lesions of the skin and some bacterial infections [13].
Our aim in this study was to evaluate the effectiveness of PDT in the inactivation of SARS-CoV-2 viral suspensions along with the treatment of SARS-CoV-2-infected green monkey kidney cells using photosensitizers such as MB and Radachlorin as medicinal drugs.
2. Materials and methods
2.1. Virus and cell
The viral strain RP/2020 SARS-CoV-2 was isolated in Vero E6 cells in early 2020 in Russia from a patient suffering from COVID-19. The virus was cultivated in a monolayer of the monkey kidney cells, Vero E6 (SRC VB “Vector”), using Minimum Essential Medium (MEM) supplemented with 5% fetal bovine serum and 40 μg/mL gentamicin sulfate. The infectious activities of the viral suspensions were determined using the 50 % tissue culture infectious dose (TCID50) method [14].
2.2. Laser treatment of viral suspension
Light exposure for the viral suspension and the infected cell culture monolayers was carried out by using continuous monoposition radiation generated using a Lahta Milon semiconductor laser generator with a wavelength λ = 662 nm. This step was carried out in a light box containing plastic tubes with viral suspensions or plastic culture vessels having a bottom area of 25 cm2 with cell culture monolayers. Two light energy dosing modes with doses of 16 J/cm2 and 40 J/cm2 were selected for the laser PDT for durations of 40 and 100 s using a laser radiation power W =350 mW. Different PS concentrations were prepared by diluting a stock 1% water solution of MB (10.0 mg/mL) and 0.35 % water solution of Radachlorin (3.5 mg/mL) in MEM.
To study the direct inactivation of SARS-CoV-2 in vitro by PDT with MB and Radachlorin, 0.2 mL of viral suspensions containing 103 50 % tissue culture infection doses (TCID50) of SARS-CoV-2 was transferred to 1.5 mL microtubes, and equal volumes of the PSs in the culture medium were added. Immediately after addition, each sample was treated with λ = 662 nm laser radiation with 16 J/cm2 or 40 J/cm2 light energy, and the Vero E6 cell monolayer cultured in microwells was immediately infected to analyze the effects on the virus.
2.3. Laser treatment of cellular monolayers
To assess the photodynamic effects on SARS-CoV-2 replication within Vero E6 cells, subconfluent monolayers in T25 (25 cm2) plastic cell culture flasks were infected with 102 to 105 TCID50 of the viral mixture. Then, the PSs were added. This addition was followed by light treatment of the culture vials with laser light at an energy density of 40 J/cm2 and using 350 mW of output power. Samples containing the added PSs without laser treatment, samples undergoing laser irradiation without the addition of PS, and samples with neither the PSs nor light treatment were used as the controls.
2.4. Inhibition of virus replication
The antiviral effects of PDT on SARS-CoV-2-containing suspensions were determined 48 h post infection (p.i.). In this regard, monolayers of infected Vero E6 cells were incubated in 96-well cell plastic plates at 37 °C in a CO2 incubator in total darkness. At 2 days p.i., virus-induced CPE was evaluated by microscopy and then confirmed by a formazan-based MTT [3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide] cell viability assay [15].
The antiviral activity of the PSs is expressed as the IC50, defined as the compound concentration producing 50 % inhibition of virus replication, as estimated by microscopic scoring of the CPE and by measuring cell viability in the formazan-based MTT assay [16]. Briefly, cells were seeded in a 96-well flat-bottom microtiter plate at a density of 5 × 104 cells/well; after 24 h of incubation, cell monolayers were infected with SARS-CoV-2 (102 TCID50/well), and dilutions of PSs were added to the cells immediately or at 3.5 h p.i.; after 48 h of incubation, the culture medium was replaced with fresh medium; 10 μL of MTT working solution (5 mg/mL in phosphate buffer solution) was added to each well, and the plate was incubated for 4 h at 37 °C; the medium was then aspirated, and the formed formazan crystals were solubilized by adding 50 μL of DMSO per well for 30 min at 37 °C; the intensity of the optical density was quantified using an ELISA plate reader at 540 nm. Cytotoxicity is expressed as the CC50, the compound concentration producing a 50 % cytotoxic effect estimated by the MTT cell viability assay.
Statistical data processing was conducted using the statistical program STATISTICA 12 (StatSoft Inc., USA). Statistical significance was determined at p < 0.05.
2.5. Biosafety
All experiments involving any infectious viral materials were conducted in a biosafety level-3 laboratory after obtaining all the national certificates and permissions required for studying SARS-CoV-2.
3. Results
3.1. Inactivation of SARS-CoV-2
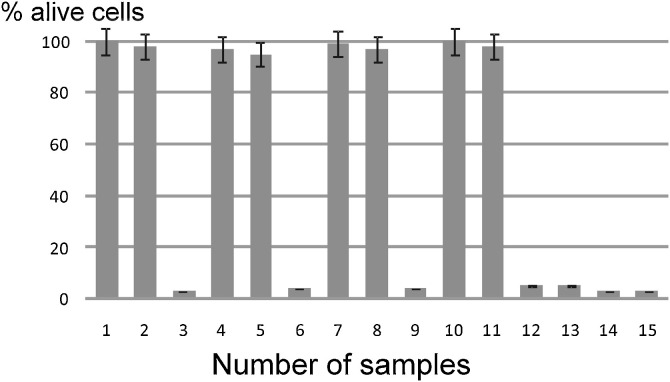
The PDT against the 103 TCID50 of SARS-CoV-2 suspensions, with light energies of 16 J/cm2 and 40 J/cm2, completely inactivated viral infectivity in the presence of PSs used in the concentration range of 1.0–10.0 μg/ml for MB and 0.5–5.0 μg/ml for Radachlorin (Fig. 1 ). Concurrently, laser treatment of the control viral suspensions without PSs and PS treatment without laser irradiation did not lead to a significant reduction in the viral CPE of the infected Vero E6 cell monolayers. Thus, these results indicate a high effectiveness of PDT in inactivating SARS-CoV-2 viral suspensions when accompanied by low concentrations of photosensitizers, such as MB and Radachlorin.
Fig. 1.
Photodynamic inactivation of SARS-CoV-2 in viral suspensions using MB and Radachlorin.
Data are the mean ± SEM of 2 independent MTT tests (3 replicates per point). % viable cells = (abssample – absblank)/(abscontrol – absblank) x 100.
1. Monolayers (2 × 105 cells in each well) were infected with a mixture containing 103 TCID50 of SARS-CoV-2, with the addition of MB at a concentration of 10.0 μg/mL, following laser treatment for 40 s at a 16 J/cm2 dose;
2. 103 TCID50 of SARS-CoV-2, 10.0 μg/mL MB, 100 s, 40 J/cm2;
3. 103 TCID50 of SARS-CoV-2, 10.0 μg/mL MB, without laser treatment;
4. 103 TCID50 of SARS-CoV-2, 1.0 μg/mL MB, 40 s, 16 J/cm2;
5. 103 TCID50 of SARS-CoV-2, 1.0 μg/mL MB, 100 s, 40 J/cm2;
6. 103 TCID50 of SARS-CoV-2, 1.0 μg/mL MB, without laser treatment;
7. 103 TCID50 of SARS-CoV-2, 5.0 μg/mL Radachlorin, 40 s, 16 J/cm2;
8. 103 TCID50 of SARS-CoV-2, 5.0 μg/mL Radachlorin, 100 s, 40 J/cm2;
9. 103 TCID50 of SARS-CoV-2, 5.0 μg/mL Radahlorin, without laser treatment;
10. 103 TCID50 of SARS-CoV-2, 0.5 μg/mL Radachlorin, 40 s, 16 J/cm2;
11. 103 TCID50 of SARS-CoV-2, 0.5 μg/mL Radachlorin, 100 s, 40 J/cm2;
12. 103 TCID50 of SARS-CoV-2, 0.5 μg/mL Radachlorin, without laser treatment;
13. 103 TCID50 of SARS-CoV-2, laser irradiation, 40 s, 16 J/cm2;
14. 103 TCID50 of SARS-CoV-2, laser irradiation, 100 s, 40 J/cm2;
15. 103 TCID50 of SARS-CoV-2, without laser treatment.
3.2. Treatment of SARS-CoV-2 infected cells
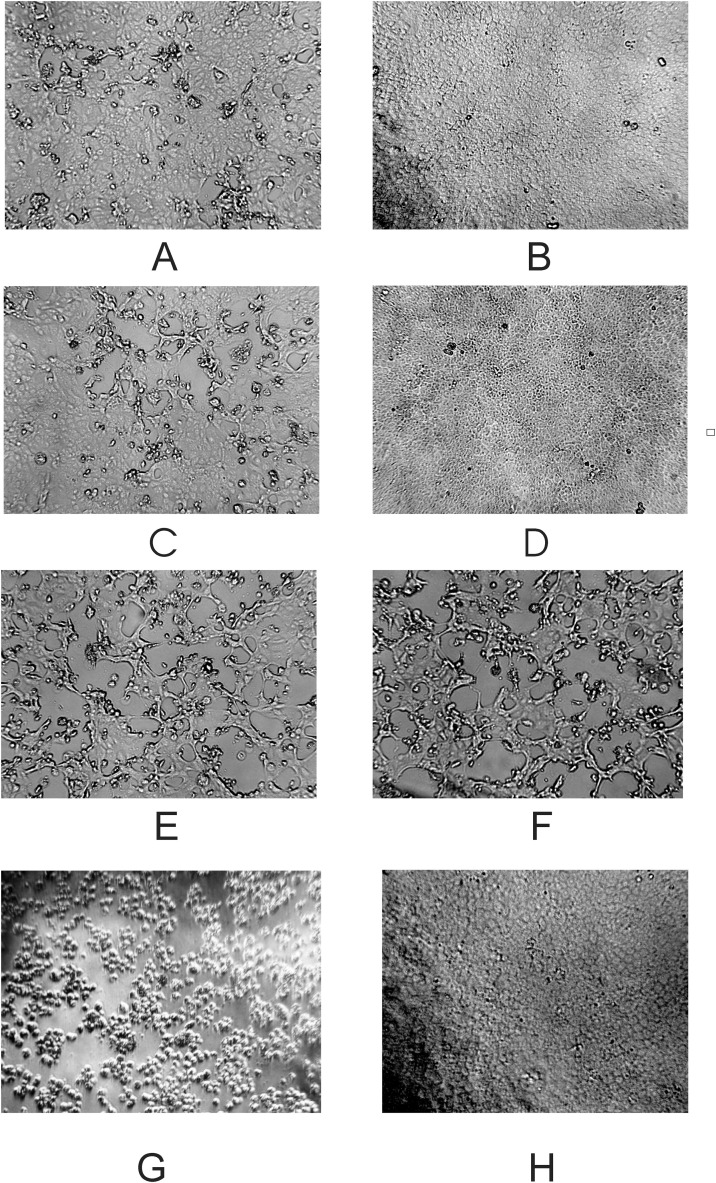
To assess the effect of photodynamic therapy on SARS-CoV-2 replication within Vero E6 cells, subconfluent monolayers in cell culture flasks were infected with 102 to 105 TCID50 of the viral mixture, followed by the addition of PSs and PDT. Fig. 2 shows microphotographs of the cellular monolayers 2 days post infection and PDT. The cell monolayers infected with 104 and 103 TCID50 of SARS-CoV-2 were fully protected after PDT with MB, whereas when cell monolayers were infected with 105 TCID50, we observed only single foci of viral lesions. PDT in the presence of Radachlorin also fully inhibited viral replication in the cells infected with 103 TCID50 and provided more than 50 % cell protection against 104 TCID50. The cell monolayers infected with 103 TCID50 in the presence of PSs without laser treatment were protected by approximately 15–25 %. We observed complete CPE for control monolayers infected with SARS-CoV-2 in similar doses. Thus, PDT accompanied by MB and Radachlorin effectively inhibits viral replication in SARS-CoV-2-infected cells and can completely protect cellular monolayers from viral infections.
Fig. 2.
Microphotographs of Vero E6 monolayers* (magnification × 100) 48 h after infection with SARS-CoV-2 and having undergone PDT.
* - 3 flasks/images per group.
A) Vero E6 cells infected with 105 TCID50 of the SARS-CoV-2 suspension, followed by treatment with 1.0 μg/mL MB and laser irradiation for 100 s, 40 J/cm2;
B) 104 TCID50 of SARS-CoV-2, 1.0 μg/mL MB, 100 s, 40 J/cm2;
C) 104 TCID50 of SARS-CoV-2, 0.5 μg/mL Radachlorin, 100 s, 40 J/cm2;
D) 103 TCID50 of SARS-CoV-2, 0.5 μg/mL Radachlorin, 100 s, 40 J/cm2;
E) 103 TCID50 of SARS-CoV-2, 1.0 μg/mL MB, without laser treatment;
F) 103 TCID50 of SARS-CoV-2, 0.5 μg/mL Radahlorin, without laser treatment;
G) 103 TCID50 of SARS-CoV-2;
H) Uninfected control.
3.3. Antiviral activity of PSs
A study of the antiviral activity of PSs was conducted in the absence of light exposure. The antiviral activity of PSs was determined in two variants. In the first variant, Vero E6 monolayers were infected with 102 TCID50 of SARS-CoV-2, and PSs were immediately added to the cells. In the second, PSs were added to infected cells 3.5 h post infection. The results demonstrated that the PSs exerted significant antiviral activity in both cases (Table 1 ). The IC50 (50 % effective inhibitory concentration) was 0.22 μg/mL for MB, and the therapeutic index (the ratio of CC50 to IC50) was more than 450.
Table 1.
Antiviral activity of MB and Radachlorin in Vero E6 cells without laser irradiation.
| Compounds | PS added to cells along with SARS-CoV-2 (IC50 μg/mL) | PS added to cells 3.5 h post infection with SARS-CoV-2 (IC50 μg/mL) | CC50 μg/mL |
|---|---|---|---|
| MB | 0.22 ± 0.07 | 0.66 ± 0.23 | 100 ± 24.0 |
| Radachlorin | 0.33 ± 0.09 | 2.0 ± 0.60 | 50 ± 15.0 |
| T-705 and T-1105 | ≥ 60 | NT | > 250* |
Note: Data are the mean ± SEM of 3 independent tests (3 replicates for one test per concentration; the ranges of concentrations tested were 150−0.06 μg/mL for MB and Radachlorin and 60−0.02 μg/mL for T-705 and T-1105). Antiviral activity is expressed as the IC50, defined as the compound concentration producing 50 % inhibition of virus replication, as estimated by microscopic scoring of the CPE and by measuring cell viability in the formazan-based MTT assay. Cytotoxicity is expressed as the CC50, the compound concentration producing 50 % cytotoxic effect estimated by the MTT cell viability assay.
The T-705 and T-1105 compounds were kindly provided by Prof. Tikhonov A.Y. (N.N. Vorozhtsov Novosibirsk Institute of Organic Chemistry of the SB RAS) as potential inhibitors for viral RNA-dependent RNA polymerase of influenza virus. *the CC50 value [17]. NT - not tested.
Interestingly, the antiviral activity of MB and Radachlorin was also detected when PSs were added to cells after a significant amount of time post infection (IC50 - 0.66 and 2.0 μg/mL, respectively). This result excludes the direct inactivation of SARS-CoV-2 viral particles with MB and Radahlorin within the extracellular environment since the internalization of these viral particles into the cells had already been completed, and new viral particles had not yet formed. The CC50 for the PSs was significantly higher than the corresponding IC50, while the therapeutic indexes ranged from 25 to 150. These results indicate that PSs could potentially be used for the successful treatment of coronavirus infection. T-705 or T-1105 compounds [17] that are capable of inhibiting viral RNA-dependent RNA polymerase were used in these experiments as comparative controls. Their antiviral activities against SARS-CoV-2 were at least 100 times lower than those of MB and Radachlorin.
4. Discussion
A hypothesis about the potential efficacy of PDT against SARS-CoV-2 was formulated relatively recently, and a short report on the application of PDT in humans has also been published [18,19]. We attempted to investigate the possibility of using PDT to inactivate SARS-CoV-2 and inhibit its replication within a monolayer of virus-sensitive green monkey kidney cells. Experimental studies have made it possible for the first time to detect the high antiviral activity of MB and Radachlorin in relation to SARS-CoV-2 by using PDT in vitro at 662 nm along with PSs at concentrations 100–1000 times lower than that in their medicinal form, which is widely applied in Russia. The antiviral activity of PDT manifested as the direct inactivation of viral suspensions and provided protection to Vero E6 cells against infection by SARS-CoV-2. The absence of viral lesions in the cellular monolayers suggests that PDT completely inhibited viral replication in these highly sensitive cells.
The mechanism of the antiviral activity of PDT against SARS CoV-2 still needs to be fully clarified [5]. Typically, the antiviral effect is associated with the interaction of PSs with viral or cellular molecules. PSs are activated by light and then transfer energy to other molecules. Oxygen usually acts as an energy acceptor by attaining a singlet form and triggering a cascade of free-radical reactions that damage the biological structures of viruses as well as rapidly proliferating and malignant cells. Additionally, there is also recent evidence of possible in vitro antiviral activity for MB even in the absence of light-induced activation, as MB showed virucidal activity at low micromolar concentrations when incubated with SARS-CoV-2 [20]. Its ability to inhibit the protein-protein interaction of the SARS-CoV-2 spike protein and its receptor ACE2, which is the first critical step initiating viral attachment and entry, could be a mechanism of action contributing to such activity.
It is important to note that SARS CoV-2 is a fast-replicating coronavirus that usually causes complete cell lysis within a short duration. The large size of the viral genomic RNA (30,000 bp) can also be an important factor in explaining the high effectiveness of PDT for direct inactivation of viral suspensions. The protection of PS-treated cell monolayers against viral infection (both immediately and 3.5 h after infection) indicates that PS can completely block viral replication within infected cells during the phase of active viral synthesis. Another possibility may be the direct inactivation of viral particles when they egress from primary infected cells.
The pathogenesis of the early stages of COVID-19 is associated with the penetration of the upper respiratory tract by SARS CoV-2 and the subsequent development of viral infection in tissues of the upper and lower respiratory tracts [21]. This ensures further local viral replication within respiratory tract cells and further lymphogenic and hematogenic spread throughout the body. Moreover, the level of lung damage largely determines the severity of the disease and the outcome of the illness. These features of COVID-19 pathogenesis potentiate the use of PDT for targeted prevention and treatment of the disease. The proposed therapy may be based on PS delivery systems through irrigation and/or inhalation into the respiratory tract. The possibility of accessing both the upper and lower respiratory tracts using light radiation, especially in the red and near infrared regions of the spectrum, is also well known [22]. This suggests that PDT may become a highly effective method for the prevention and treatment of COVID-19, especially in the early stages of the disease. A fundamental advantage of this approach is that it can be used during a primary outbreak of coronavirus infection to cease the outbreak and prevent contact infection, particularly in asymptomatic patients.
Thus, the results obtained in this study suggest the prospect of using PDT for the treatment of coronavirus infection in patients. The local use of MB and Radachlorin by irrigation (washing) of the upper respiratory tract or PS delivery to the lower respiratory tract via inhalation for the inactivation and inhibition of SARS CoV-2 replication directly within the target organ (lungs) are promising potential strategies for extensive clinical use in the prevention and treatment of COVID-19.
Ethical compliance
This article does not describe any studies involving humans or animals as the test subjects.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgments
The research was supported by the Novosibirsk State University, Russia; Institute of Laser Physics of the Siberian Branch of the Russian Academy, Russia.
References
- 1.da Costa V.G., Moreli M.L., Saivish M.V. The emergence of SARS, MERS and novel SARS-2 coronaviruses in the 21st century. Arch. Virol. 2020;165:1517–1526. doi: 10.1007/s00705-020-04628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Worldometer, COVID-19 coronavirus pandemic. https://www.worldometers.info/coronavirus/ 2020 (accessed 05 December 2020).
- 4.Lai C.-C., Shih T.-P., Ko W.-C., Tang H.-J., Hsueh P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costa L., Faustino M.A., Neves M.G., Cunha A., Almeida A. Photodynamic inactivation of mammalian viruses and bacteriophages. Viruses. 2012;4:1034–1074. doi: 10.3390/v4071034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rywkin S., Ben-Hur E., Malik Z., Prince A.M., Li Y.S., Kenney M.E., Oleinick N.L., Horowitz B. New phthalocynanines for photodynamic virus inactivation in red blood cell concentrates. Photochem. Photobiol. 1994;60:165–170. doi: 10.1111/j.1751-1097.1994.tb05085.x. [DOI] [PubMed] [Google Scholar]
- 7.Käsermann F., Kempf C. Photodynamic inactivation of enveloped viruses by buckminsterfullerene. Antivir. Res. 1997;34:65–70. doi: 10.1016/S0166-3542(96)01207-7. [DOI] [PubMed] [Google Scholar]
- 8.Nikolaeva-Glomb L., Mukova L., Nikolova N., Kussovski V., Doumanova L., Mantareva V., Angelov I., Wöhrle D., Galabov A.S. Photodynamic effect of some phthalocyanines on enveloped and naked viruses. Acta Virol. 2017;61:341–346. doi: 10.4149/av_2017_313. [DOI] [PubMed] [Google Scholar]
- 9.Hamblin M.R., Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004;5:436–450. doi: 10.1039/B311900A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felber T.D., Smith E.B., Knox J.M., Wallis C., Melnick J.L. Photodynamic inactivation of herpes simplex: report of a clinical trial. J. Am. Med. Assoc. 1973;92:223–289. doi: 10.1001/jama.1973.03220030027005. [DOI] [Google Scholar]
- 11.Perdrau J.R., Todd C. The photodynamic action of methylene blue on certain viruses. Proc. Roy. Soc. Lond. B Biol. Sci. 1933;112:288–298. doi: 10.1098/rspb.1933.0011. [DOI] [Google Scholar]
- 12.Wong T.-W., Huang H.-J., Wang Y.-F., Lee Y.-P., Huang C.-C., Yu C.-K. Methylene blue-mediated photodynamic inactivation as a novel disinfectant of enterovirus 71. J. Antimicrob. Chemother. 2010;65:2176–2182. doi: 10.1093/jac/dkq301. [DOI] [PubMed] [Google Scholar]
- 13.Bredikhin D.A., Nikonov S.D., Cherednichenko A.G., Petrenko T.I. In vitro photodynamic inactivation of Mycobacterium tuberculosis by Radahlorin. Tuberc. Lung Dis. 2018;96:5–10. doi: 10.21292/2075-1230-2018-96-1-5-10. In Russ. [DOI] [Google Scholar]
- 14.Chanas A.C., Johnson B.K., Simpson D.I. Antigenic relationships of alphaviruses by a simple micro-culture cross-neutralization method. J. Gen. Virol. 1976;32:295–300. doi: 10.1099/0022-1317-32-2-295. [DOI] [PubMed] [Google Scholar]
- 15.Niks M., Otto M. Towards an optimized MTT assay. J. Immunol. Methods. 1990;130:149–151. doi: 10.1016/0022-1759(90)90309-j. [DOI] [PubMed] [Google Scholar]
- 16.Kiriliuk I.A., Svyatchenko V.A., Morozov D.A., Kazachinskaia E.I., Kiselev N.N., Bakunova S.M., Voinov M.A., Loktev V.B., Grigoryev I.A. In vitro cytotoxicity of nitroxyl radicals with respect to tumor and diploid human cells and estimation of their antiviral activity. Antibiot. Khimioter. 2012;57(1-2):3–12. In Russ. [PubMed] [Google Scholar]
- 17.Huchting J., Vanderlinden E., Winkler M., Nasser H., Naesens L., Meier C. Prodrugs of the phosphoribosylated forms of hydroxypyrazinecarboxamide pseudobase T-705 and its de-fluoro analogue T-1105 as potent influenza virus inhibitors. J. Med. Chem. 2018;61:6193–6210. doi: 10.1021/acs.jmedchem.8b00617. [DOI] [PubMed] [Google Scholar]
- 18.Almeida A., Faustino M.A.F., Neves M.G.P.M.S. Antimicrobial photodynamic therapy in the control of COVID-19. Antibiotics Basel (Basel) 2020;9:E320. doi: 10.3390/antibiotics9060320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dias L.D., Blanco K.C., Bagnato V.S. COVID-19: beyond the virus. The use of photodynamic therapy for the treatment of infections in the respiratory tract. Photodiagnosis Photodyn. Ther. 2020;31 doi: 10.1016/j.pdpdt.2020.101804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cagno V., Medaglia C., Cerny A., Cerny T., Tapparel C., Cerny E. 2020. Methylene Blue Has a Potent Antiviral Activity Against SARS-CoV-2 in the Absence of UV-activation in Vitro. BioRxiv, 2008.2014.251090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J., Zheng X., Tong Q., Li W., Wang B., Sutter K., Trilling M., Lu M., Dittmer U., Yang D. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS-CoV, MERS-CoV, and 2019-nCoV. J. Med. Virol. 2020;92:491–494. doi: 10.1002/jmv.25709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Hussein A., Manoto S.L., Ombinda-Lemboumba S., Alrowaili Z.A., Mthunzi-Kufa P.A. Review of chemotherapy and photodynamic therapy for lung Cancer treatment. Anticancer Agents Med. Chem. 2020 doi: 10.2174/1871520620666200403144945. [DOI] [PubMed] [Google Scholar]