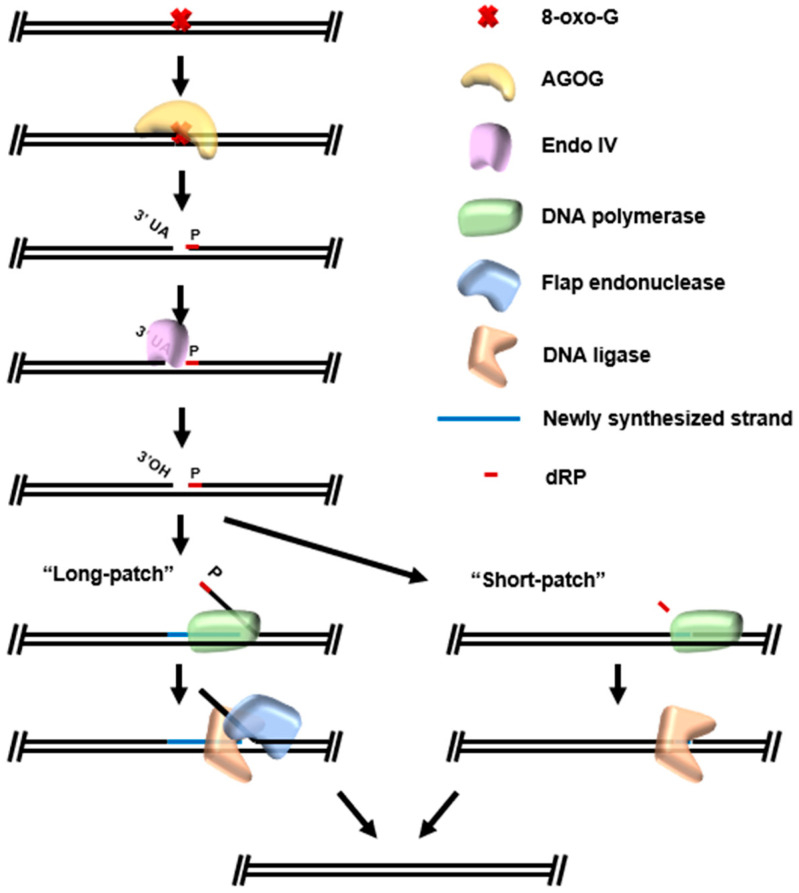
Figure 7.
Reconstituted archaeal base excision repair from Thermococcus kodakarensis. AGOG recognizes 8-oxo-G modifications and acts as a bifunctional glycosylase, both excising the damaged base and cleaving the DNA backbone at the site of damage. The resulting substrate contains a 3′ unsaturated aldehyde (UA) and 5′ dRP. Damage repair is initiated by the activity of Endonuclease IV, which converts the 3′-UA to an extendable 3′-hydroxyl group. In long-patch base excision repair (BER), strand displacement activity of DNA polymerase during synthesis is used in tandem with flap endonuclease and DNA ligase to conclude repair. In short-patch BER, dRP lyase activity intrinsic to DNA polymerase simply removes the dRP moiety while synthesizing the correct base from the undamaged strand, and DNA ligase seals the nick.