FIGURE 5.
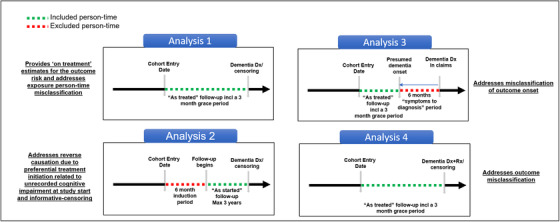
Specific design considerations to address unique challenges in using observational health‐care data sources to evaluate associations between medication use and the risk of Alzheimer's disease and related dementia (ADRD). Analysis 1 uses an “As‐treated” follow‐up approach, in which follow‐up will start on the day after the cohort entry date and censoring at follow‐up will occur at treatment discontinuation or switch (to comparator treatment) to provide estimates of “on treatment” risk of newly occurring ADRD for patients initiating the exposure of interest. Analysis 2 uses an “As‐started” follow‐up approach incorporating a 6‐month “induction” period, in which patients will be followed for a maximum of 3 years regardless of subsequent treatment changes or discontinuation, similar to an intent‐to‐treat approach in randomized trials. Analysis 3 incorporates a 6‐month “symptoms to diagnosis” period in which outcome date assigned is 6 months before the first recorded ADRD date to incorporate a “symptoms to diagnosis” period to address the potential bias due to misclassification of ADRD onset. Analysis 4 uses an alternative outcome definition including at least one diagnosis code combined with a prescription for a symptomatic treatment (donepezil, galantamine, rivastigmine, and memantine) to improve the specificity of outcome definition
