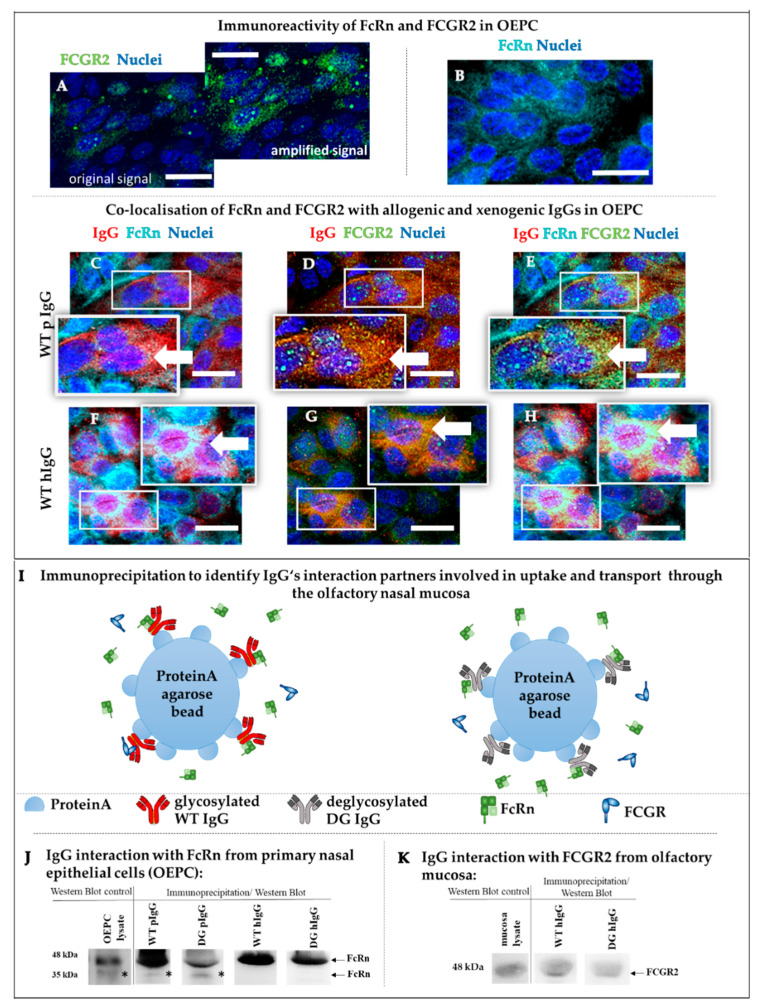
Figure 5.
Analysis of IgG transporter expression and protein–protein interaction. (A–H) Co-localization study of FcRn (B,C,F) and FCGR2 (A,D,G) with porcine (D,E) and human (F–H) IgG in primary cells from olfactory epithelium (OEPC) (representative data are shown, N = 3, n = 3). (A) To visualize the low amount of FCGR2 (original), the intensity was highly increased (increased intensity) to evaluate co-localization with FcRn and IgG. FCGR2 expression was found to be mainly intracellular. (B) FcRn was found to be intracellular and on the cell’s surface with sufficient immunoreactivity. (C–E) Co-localization of FCGR2 (green), FcRn (cyan) and pIgG (red). (F–H) Co-localization of FCGR2 (green), FcRn (cyan) and hIgG (red). Scale bar: 20 µm, arrows mark sites of co-localization. (I) Underlying principle of the immunoprecipitation (IP) to evaluate the binding of wild-type (WT hIgG) and deglycosylated human IgG (DG hIgG) and porcine IgG (WT pIgG, DG pIgG) to the porcine FcRn. (J) IP study of FcRn interaction with wild-type and deglycosylated allogenic pIgG and xenogenic hIgG using primary cell lysate (OEPC, origin: porcine nasal mucosa, olfactory region) (N = 3). IgGs were incubated with OEPC cell lysate and captured using Protein A agarose beads. The resulting bands could not be quantified due to high batch-to-batch variations in the cell lysates. * The additional band in the line of the WT and DG pIgG is caused by different glycovariants of the porcine FcRn [14]. hIgGs seem to bind only to one of the variants. (K) Interaction study (IP) of WT hIgG and DG hIgG with FCGR2 using whole mucosa lysate from c.n. media.