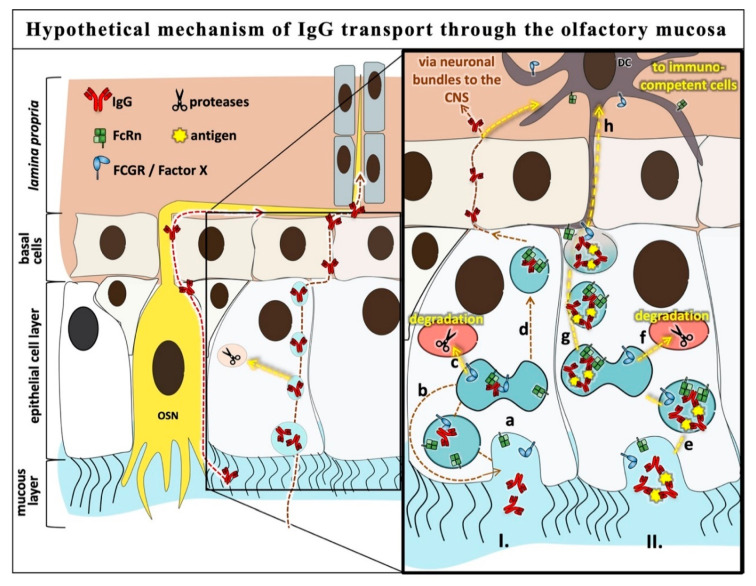
Figure 6.
Cross-binding hypothesis: hypothetical mechanism of IgG transport through the olfactory mucosa. I. (a) Uptake of monomeric IgGs was presumably via pinocytosis, but FcRn was also found at the apical surface [13] and vesicle transport to the sorting compartment. (b) Cross-binding of IgG with FcRn and a second Fc receptor such as FCGR2, which might be factor X as suggested [52]; recycling back to the apical surface or (c) lysosomal degradation. Alternatively, a second FcRn replaces FCGR2/factor X and (d) the monomeric IgG is transcytosed to the lamina propria/basolateral side. II. (e) Uptake of immune complexes similar to monomeric IgG and vesicle transport to the sorting compartment. Binding to FCGR2/factor X is not possible due to steric hindrance; therefore, double binding of FcRn to one Fc part is possible. The complex can either (f) be degraded in the lysosome or (g) transcytosed to the lamina propria and (h) taken up by dendritic cells (DC) via different FCGRs or FcRn. Further studies are needed to confirm the role of FCGR2 in IgG trafficking in the airway epithelia. Due to limitations of the commercially available anti-porcine FCGR2 antibodies, we could not distinguish between FCGR2a and FCGR2b. Nevertheless, other studies suggest FCGR2b to be expressed in epithelial cells.