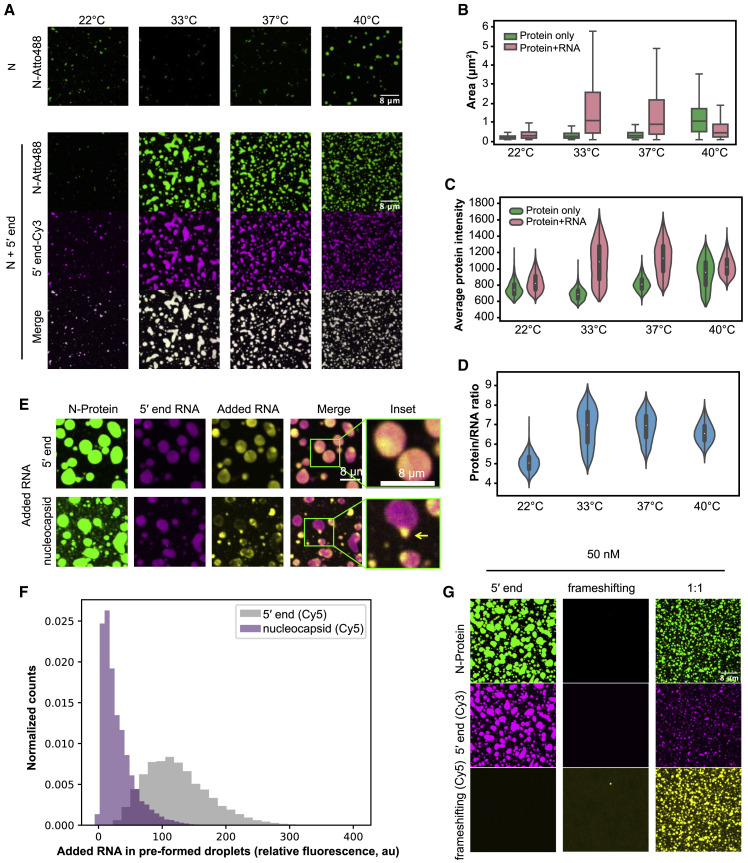
Figure 2.
N-Protein/Viral RNA Condensates Have Temperature- and RNA Sequence-Dependent Material Properties
(A) N-protein (green) alone phase separates in a temperature-dependent manner (4 μM) (upper panel). Temperature dependence is shifted when viral 5′ end RNA (25 nM) (magenta) is present (lower panel).
(B) Quantification of droplet area from (A).
(C) Quantification of average protein signal from (A) based on fluorescence intensity.
(D) Quantification of protein/RNA ratio based on fluorescence intensity from (A).
(E) Sub-genomic nucleocapsid RNA is excluded from preformed 5′ end droplets. 5′ end (yellow, upper panel) is recruited into preformed 5′ end/N-protein droplets (pink and green) but nucleocapsid RNA (yellow, lower panel) is not efficiently recruited and forms separate condensates.
(F) Quantification of (E) showing intensity of second RNA added to preformed droplets. Nucleocapsid RNA (purple) has lower distribution of signal than 5′ end RNA (gray) in regions with high preformed 5′ end RNA signal.
(G) Mixing 5′ end and frameshifting region RNAs makes N-protein condensates with intermediate properties. Left: 5′ end (magenta) and N-protein (green) produced condensates. Middle: frameshifting region (yellow) and N-protein did not produce condensates. Right: Combination of 5′ end and frameshifting region produced smaller condensates than 5′ end alone. Scale bar, 8 μm unless otherwise noted. Violin plots are scaled to have equal widths. Outliers not shown.