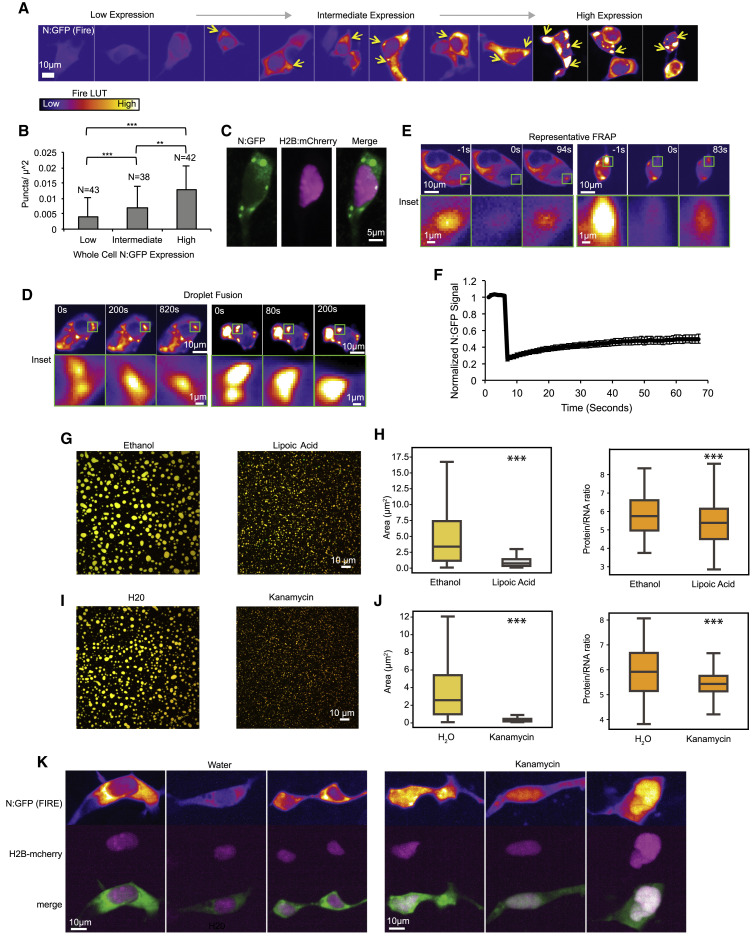
Figure 6.
N-Protein Phase Separates in Mammalian Cells and Can Be Disrupted by Small Molecules
(A) N-protein: GFP forms concentration-dependent condensates in HEK293 cells. The fire LUT represents low signal intensity in purple and high signal intensity in yellow. Yellow arrows indicate presence of condensates. Scale bar is 10 μm.
(B) Condensates per μ2 increased significantly with N:GFP expression level. ∗∗p < 0.01 ∗∗∗p < 0.001.
(C) N:GFP shown in green (both diffuse and punctate) was excluded from nuclei (marked with H2B:mCherry shown in magenta) of HEK293 cells. Scale bar is 5 μm.
(D) N:GFP (Fire LUTs) condensates fused in the cytoplasm of HEK293 cells. Top panel: representative cells with 10 μm scale bar. Bottom panel: enlarged of fusion event. Scale bar, 1 μm.
(E) N:GFP condensates recovered partially after FRAP. Top panel shows representative condensate FRAP. Scale bar is 10 μm. Bottom panel: enlargement of N:GFP condensate. Scale bar, 1 μm.
(F) Condensate N-protein exchanges with cytosolic N-protein. Condensates recovered to 24% within 1 min. Error bars show standard error from n = 18 condensates.
(G) 2.38 μg/mL lipoic acid partially prevents N-protein/frameshifting region RNA LLPS relative to ethanol vehicle. Images show merge of protein (green) and RNA (red) signals.
(H) For lipoic acid, size and protein/RNA ratio is reduced relative to vehicle. Left, quantification of condensate area depicted in (G) and right quantification of protein/RNA ratio.
(I) 0.5 mg/mL kanamycin partially prevents N-protein/frameshifting region RNA LLPS relative to water vehicle. Images show merge of protein (green) and RNA (red) signals.
(J) For kanamycin, size and protein/RNA ratio is reduced relative to vehicle. Left, quantification of condensate area depicted in (I) and right quantification of protein to RNA ratio.
(K) 5 mg/mL kanamycin causes relocalization of N:GFP (Fire LUTs) to the cell nucleus (magenta H2B:mCherry signal) in 37% of treated cells (n = 105, 0% in H2O, n = 100). Scale bar, 10 μm unless otherwise noted.