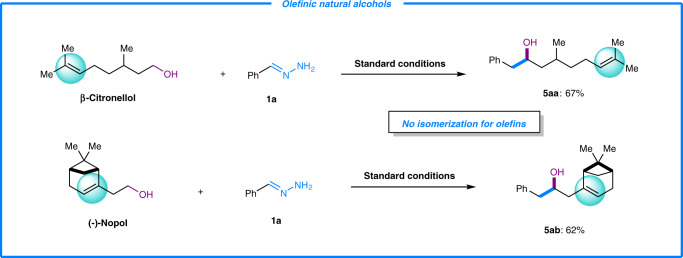
Fig. 4. Reactivity of olefinic natural alcohols.
Standard reaction conditions: 1a (0.6 mmol), 2a (0.2 mmol), Ru(PPh3)3Cl2 (5 mol %), ligand (5 mol %) and K3PO4 (2 equiv.) in 2-MeTHF at 70 °C under N2 atmosphere for 24 h. See Supplementary Information (SI) for details. Some naturally available olefinic alcohols also reacted efficiently, in which the olefin did not isomerize with the alcohol being the only reaction site. These two examples demonstrated the synthetic potentials, attributed to the special tolerance to olefins by the ruthenium catalyst.