Table 2.
Investigation of reaction conditions under oxidant-free systema.
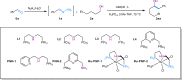 | |||||
|---|---|---|---|---|---|
| Entry | Catalyst | Ligand | K3PO4 (equiv) | Solvent (mL) | Yield |
| 1 | Ru(dppf)(en)Cl2 | — | 2 | 0.2 | 22 |
| 2 | Ru(PPh3)3Cl2 | L1 | 2 | 0.2 | 40 |
| 3 | Ru(PPh3)3Cl2 | L2 | 2 | 0.2 | 66 |
| 4 | Ru(PPh3)3Cl2 | L3 | 2 | 0.2 | 73 |
| 5 | Ru(PPh3)3Cl2 | L3 | 1.5 | 0.2 | 66 |
| 6 | Ru(PPh3)3Cl2 | L3 | 2 | 0.3 | 71 |
| 7 | Ru(PPh3)3Cl2 | L3 | 2.5 | 0.3 | 64 |
| 8 | Ru(PPh3)3Cl2 | L3 | 2 | 0.5 | 70 |
| 9b | Ru(PPh3)3Cl2 | L3 | 2 | 0.5 | 74 |
| 10b | Ru(PNN-1)(PPh3)Cl2 | — | 2 | 0.5 | 36 |
| 11b | Ru(PPh3)3Cl2 | L4 | 2 | 0.5 | 32 |
| 12b | Ru(PNN-2)H(CO)Cl2 | — | 2 | 0.5 | 17 |
| 13b | Ru-PNP-1 | — | 2 | 0.5 | 73 |
| 14b | Ru-PNP-2 | — | 2 | 0.5 | 74 |
aReaction conditions: 1a (0.7 mmol), 2a (0.2 mmol), Ru(PPh3)3Cl2 (5 mol %), ligand (5 mol %), K3PO4, 2-Me-THF at 70 °C under N2 atmosphere. See Supplementary Information (SI) for details. 1H NMR yield was determined using 1,3,5-trimethoxylbenzene as an internal standard. A ‘trace’ amount of product was noted when the desired product was not clearly detected.
b1a (0.6 mmol) was used.
