Figure 1.
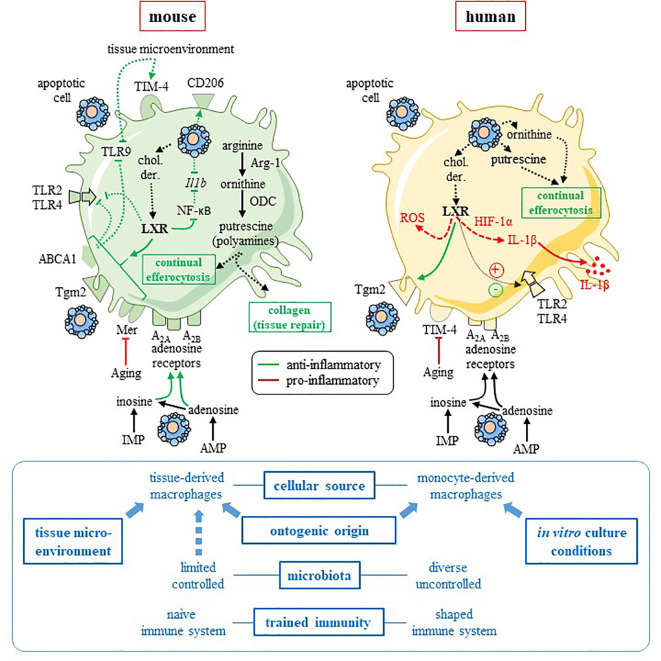
Comparison of mouse and human pro-resolving macrophages. This figure summarizes the data discussed in the text, and focuses on two pathways involved in mouse macrophage anti-inflammatory reprogramming, namely the L-arginine metabolism and the LXR pathway. Interspecies heterogeneity is reported for these two pathways. Mouse pro-resolving macrophages (left-hand side) are characterized by arginase-1 (Arg-1) and uses the arginine pathway to stimulate tissue repair and “continual” efferocytosis. Efferocytosis and tissue microenvironment imprint an anti-inflammatory profile with an increased expression of M2 receptor CD206 (13) and efferocytic receptor TIM-4 (14), as well as a downregulation of pro-inflammatory Tlr9 (14) and Il1b (13) genes. Cholesterol derivatives (chol. der.) issued from apoptotic cells may promote the LXR pathway that is responsible for upregulation of efferocytic receptor Mer (7) and an anti-inflammatory response, including inhibition of NF-κB (31) and TLR2, 4 and 9 signaling pathway via the LXR target factor, ABCA1 (32). LXR upregulates Tgm2 in mouse macrophages (33). Metabolites released from apoptotic cells (i.e., inosine-monophosphate and adenosine-monophosphate) imprint an anti-inflammatory profile (11), possibly via A2A and A2B adenosine receptors, which are highly expressed and functional in mouse cells. Thus, in mouse macrophages, adenosine A2A receptors are the primary target of apoptotic cell-derived adenosine and these receptors mediated apoptotic-cell induced immune suppression (34, 35). Aging affects efferocytosis efficacy by downregulating Mer receptor (36). The picture for human macrophages (right-hand side) is more complicated and data remain incomplete. While apoptotic-cell derived ornithine and putrescine participates in “continual” efferocytosis and the anti-inflammatory program of human pro-resolving macrophages (10). LXR activation leads to anti-inflammatory functions [with upregulation of efferocytic receptor Tgm2 (37) and inhibition of TLR4 signaling (38)] and pro-inflammatory functions (15, 16, 38) [with the production of IL-1β via HIF-1α (15), as well as ROS (38)]. Human cells are less receptive to inosine and adenosine with reduced expression and function of adenosine receptors in comparison to mouse cells (39). Aging disturbs efferocytosis efficacy and the resolution of inflammation by downregulating TIM-4 receptor (36). Factors that may explain interspecies differences are written in blue font on the bottom of the figure. Blue arrows mean an influence of a given factor. Some of these factors affect rather mouse macrophages (i.e., tissue microenvironment) and others human macrophages (e.g., in vitro culture conditions). → (or plus) and ⊣ (or minus) symbols mean stimulation and inhibition, respectively. Red color means pro-inflammatory and green color pro-resolving. Solid line identifies a direct effect, while dotted line an indirect (or supposed) effect. ABCA1, adenosine triphosphate-binding cassette A1; AMP, adenosine-monophosphate; Arg-1, arginase-1; chol. der., cholesterol derivatives; HIF-1α, hypoxia-inducing factor-1α; Il1b, interleukin-1 beta gene; IL-1β, interleukin-1β; IMP, inosine-monophosphate; LXR, Liver X receptor; NF-κB, nuclear factor-kappa B; ODC, ornithine decarboxylase; ROS, reactive oxygen species; Tgm2, transglutaminase-2; TIM4, T-cell immunoglobulin and mucin domain containing 4; TLR, Toll-like receptor. This figure was depicted, in part, by using Servier Medical Art, https://smart.servier.com/.