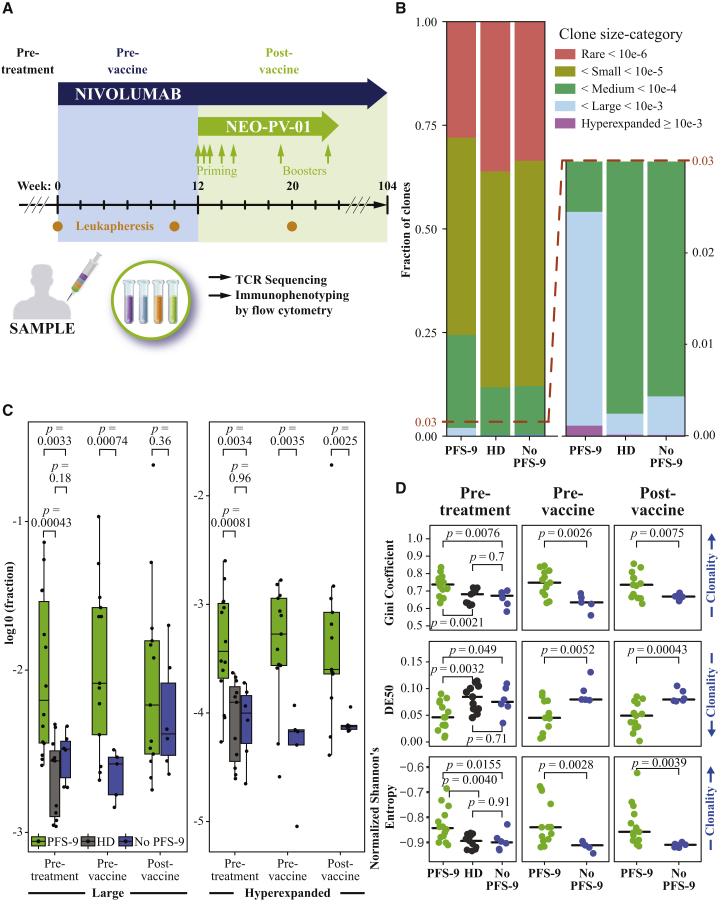
Figure 1.
Patients with Lack of Progression at 9 Months Have a Higher Peripheral TCR Repertoire Clonality prior to and throughout Study Treatment
(A) Treatment schedule outline of the single-arm clinical trial of nivolumab plus personalized neoantigen vaccine (NEO-PV-01). Timings are indicated for nivolumab (blue arrow), personalized vaccine (NEO-PV-01, green arrow), and leukaphereses (orange dots). Leukaphereses from three time points (pre-treatment, pre-vaccine, and post-vaccine) are used for CD3+ T cell isolation for TCR-α/β sequencing and PBMCs for immunophenotyping by flow cytometry.
(B) The proportion of clones belonging to each clone size category, averaged across patients (or HDs), and time points. An inset focusing on larger bins is provided (right). The legend defines frequency-based categories.
(C) The (log–) fraction of clones belonging to the large (left) or hyperexpanded (right), for patients with and without PFS-9 at each time point or HDs. Boxplots indicate 25%, 50%, and 75% percentiles, and whiskers extend to the smallest/largest value within 1.5 times the interquartile range. p values are derived from a two-tailed Student’s t test.
(D) The skewedness of the TCR-β repertoire frequency distribution measured by the Gini coefficient, DE50, and normalized Shannon’s entropy of each HD and patient across time points. The black line indicates median. p values are derived from a two-tailed Student’s t test.