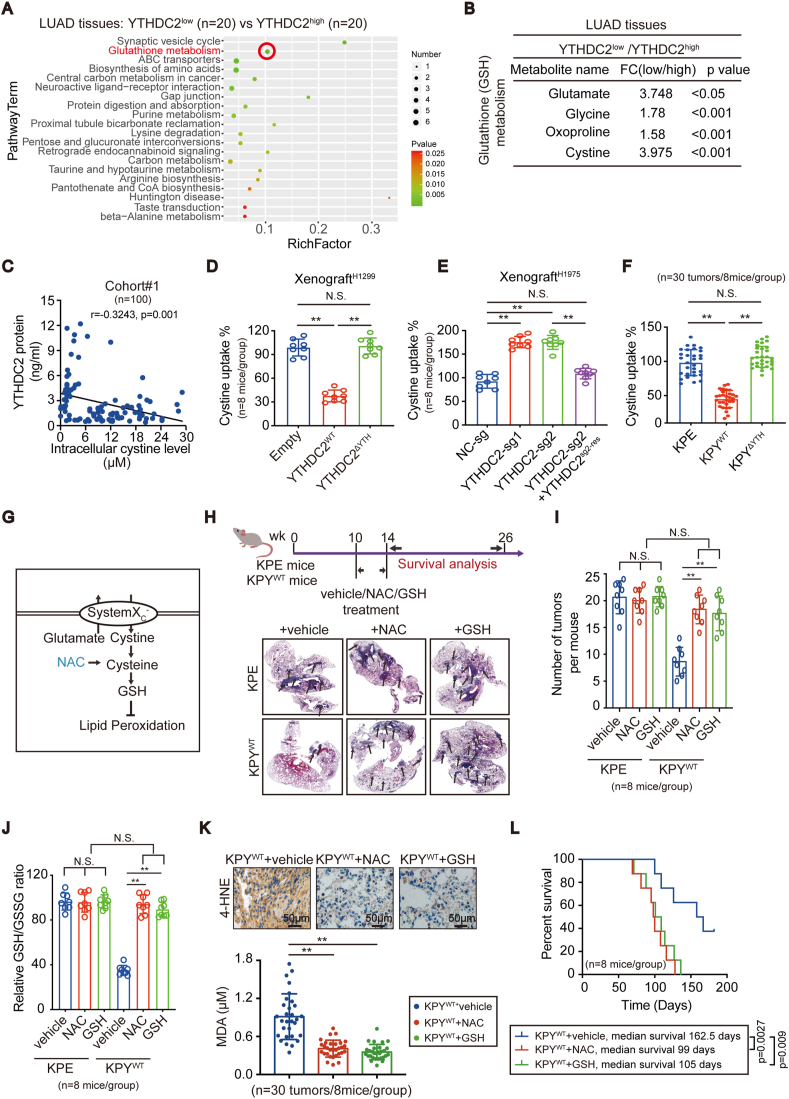
Fig. 3.
YTHDC2 suppressed cystine uptake and downstream antioxidant program in LUAD. (A, B) Statistics of top 20 enriched KEGG pathways, and alterations in metabolites involved in GSH metabolism, as measured by metabolomics in YTHDC2low (n = 20) and YTHDC2high (n = 20) LUAD tissues. (C) Correlation between intracellular cystine level and YTHDC2 protein in LUAD tissues from cohort #1 (n = 100, pearson analysis, p = 0.001). (D–F) Cystine uptake in xenografts (n = 8 per group) generated by H1299 cells with YTHDC2WT or YTHDC2ΔYTH overexpression, H1975 cells with or without YTHDC2 knockout and reconstitution, and tumors from KPE/KPYWT/KPYΔYTH mice (n = 30 tumors from 8 mice per group), as detected by L-14C-cystine (0.2 μCi/mL). (G) Schematic presentation of antioxidant program from cystine uptake to lipid peroxidation. (H–J) Schematic illustration of drug treatment strategy. Representative H&E staining of lungs bearing tumors (black arrows indicate tumors), quantification of tumors and relative GSH/GSSG ratio in KPE and KPYWT mice (n = 8 per group) administrated with vehicle, NAC (100 mg/kg/day) or GSH (100 mg/kg/day) for 4 weeks. (K, L) 4-HNE, MDA (n = 30 tumors from 8 mice per group), and survival curves in KPYWT mice (n = 8 per group) administrated with vehicle, NAC (100 mg/kg/day) or GSH (100 mg/kg/day), as determined by IHC, lipid peroxidation assays, and the Kaplan-Meier method (log-rank tests, vehicle versus NAC: p = 0.0027, vehicle versus GSH: p = 0.009), scar bar 50 μm. Statistical analysis was performed using one-way ANOVA (D-F, I-K). Data are means ± SEMs, **p < 0.01, N·S.: no significant.