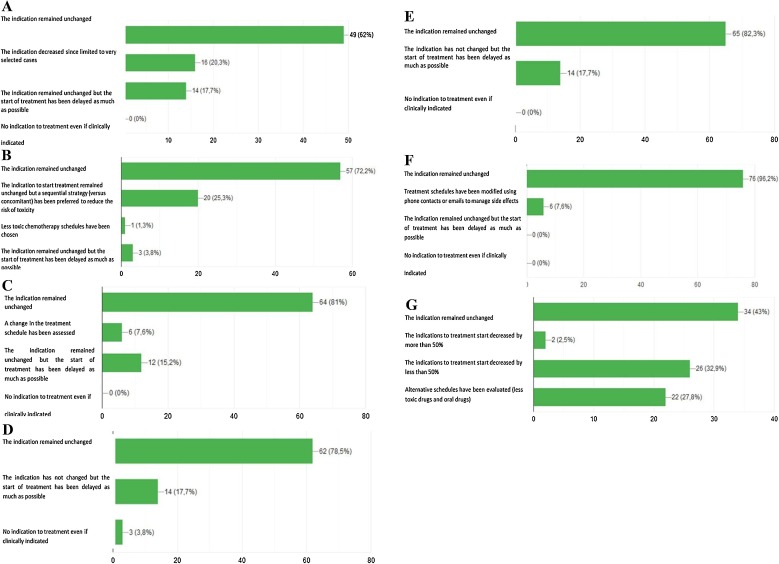
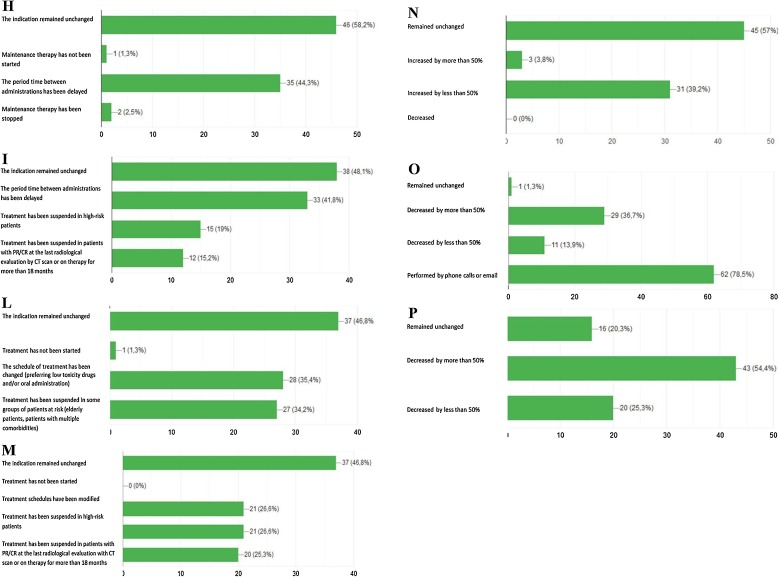
Fig. 2.
Schematic representation of the responses to the following survey’s questions: A Has the indication to adjuvant treatment been modified for surgically resected NSCLC patients? B Has the indication to concurrent chemo-radiotherapy been modified for NSCLC patients with unresectable locally advanced disease?. C Has the indication to first-line chemotherapy been modified for patients with metastatic NSCLC?. D Has the indication to first-line chemo-immunotherapy been modified for patients with metastatic NSCLC?. E Has the indication to first-line single agent immunotherapy been modified for patients with metastatic NSCLC?. F Has the indication to first-line targeted therapies been modified for patients with metastatic NSCLC? G Has treatment indication to first line chemotherapy or immunotherapy been modified in elderly patients (over 75 years) with non-oncogene addicted metastatic NSCLC?. H Has treatment indication to manteinance therapy benn modifed for patients with metastatic NSCLC candidate to start/continue maintenance chemotherapy (pemetrexed). I Has treatment indication for metastatic NSCLC patients undergoing first line immunotherapy been modified?. L Has treatment indication to second line chemotherapy been modified fro metastatic NSCLC patients?. M Has treatment indication to second line immunotherapy been modified for metastatic NSCLC patients?. N Has the indication to interrupt active treatment and start best supportive care been modified in metastatic NSCLC patients?. O Has follow-up management for NSCLC patients been modified?. P Has NSCLC patients’ accrual in ongoing clinical trials been modified?.