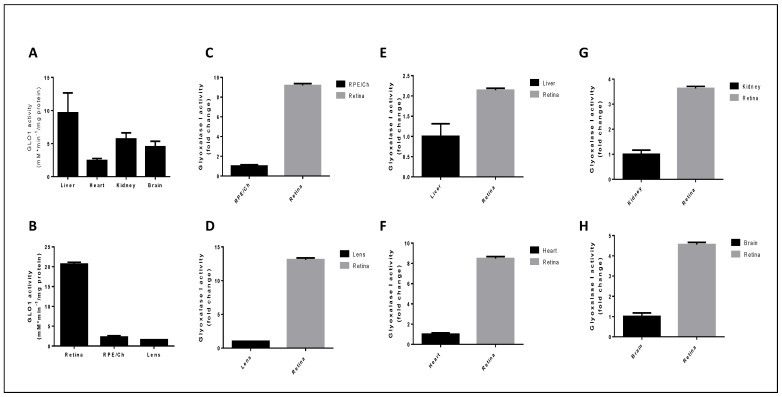
Figure 5.
Evaluation of glyoxalase system activity in ocular and non-ocular tissues. Glyoxalase I activity was determined spectrophotometrically using 1 mL quartz cuvettes by following the initial rate of formation of S-D-lactoylglutathione. The assay mixture containing the glycating reagent MG and reduced GSH was equilibrated at room temperature for 10 min, to ensure hemithioacetal formation. The reaction was initiated by the addition of 20 μg of cytosolic extract and the A240 was monitored immediately and over the course of 5 min. The reaction rate was determined by following the increase in absorbance at 240 nm for which Δε240 = 2.86 mM−1 cm−1. (A,B) Glyoxalase I activity was assayed in (A) non-ocular tissues and (B) ocular tissues and activity was expressed as milliunits per milligram of protein where one unit of GLO1 activity was the amount of enzyme which catalyzes the formation of 1 μmol S-D-lactoylglutathione per min under assay conditions. (C–H) Retinal glyoxalase I activity was compared to (C) RPE/choroid, (D) lens, (E) liver, (F) heart, (G) kidney, and (H) brain. Fold change was calculated relative to each tissue and values represent the mean ± standard error of the mean of 4 independent experiments from the GLO1 activity assay.